Calculate the bond length of tritium chloride-35 (T3CI). Give your answer in angstrom. (Round your answer to 4 decimal places.) Use the constants given below. Reduced mass of T35CI: u = 2.7766 amu Be for TCI (determined by FT-IR spectroscopy): Be =3.767 cm1 %3D C: 299 800 000 m/s h: 6.626x1034 Js
Calculate the bond length of tritium chloride-35 (T3CI). Give your answer in angstrom. (Round your answer to 4 decimal places.) Use the constants given below. Reduced mass of T35CI: u = 2.7766 amu Be for TCI (determined by FT-IR spectroscopy): Be =3.767 cm1 %3D C: 299 800 000 m/s h: 6.626x1034 Js
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.23PAE
Related questions
Question
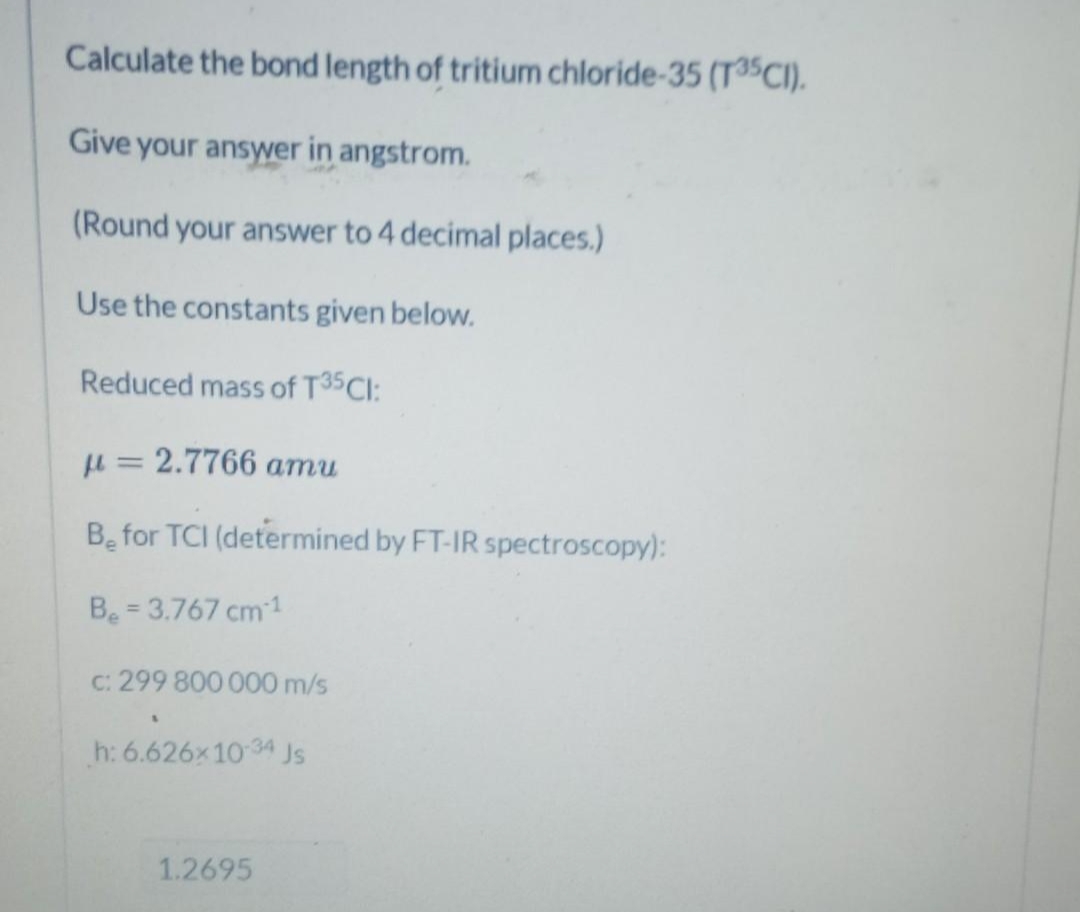
Transcribed Image Text:Calculate the bond length of tritium chloride-35 (T35CI).
Give your answer in angstrom.
(Round your answer to 4 decimal places.)
Use the constants given below.
Reduced mass of T35CI:
u = 2.7766 amu
Be for TCI (determined by FT-IR spectroscopy):
Be = 3.767 cm1
%3D
C: 299 800 000 m/s
h: 6.626x10 34
Js
1.2695
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning