Select the single best answer. Consider the following data: Metal Al Cu Mass (g) 30 10 Specific heat (J/g C) 0.900 0.385 Temperature (* C) 60 40 When these two metals are placed in contact, which of the following will take place? Oa. No heat will flow in either direction. Ob.Heat will flow from Al to Cu because Al has a larger specific heat. Oc. Heat will flow from Al to Cu because Al has a larger mass. Od. Heat will flow from Al to Cu because Al is at a higher temperature. e. Heat will fiow from Cu to Al because AI has a larger heat capacity.
Select the single best answer. Consider the following data: Metal Al Cu Mass (g) 30 10 Specific heat (J/g C) 0.900 0.385 Temperature (* C) 60 40 When these two metals are placed in contact, which of the following will take place? Oa. No heat will flow in either direction. Ob.Heat will flow from Al to Cu because Al has a larger specific heat. Oc. Heat will flow from Al to Cu because Al has a larger mass. Od. Heat will flow from Al to Cu because Al is at a higher temperature. e. Heat will fiow from Cu to Al because AI has a larger heat capacity.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 109AE: A sample of nickel is heated to 99.8C and placed in a coffee-cup calorimeter containing 150.0 g...
Related questions
Question
sub= 18 help
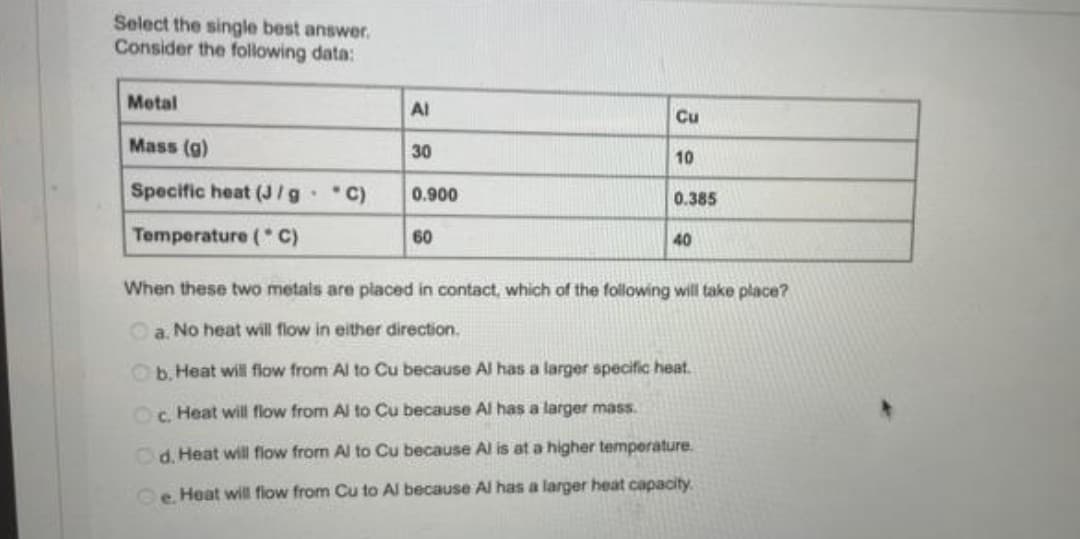
Transcribed Image Text:Select the single best answer.
Consider the following data:
Metal
Al
Cu
Mass (g)
30
10
Specific heat (J/g C)
0.900
0.385
Temperature ( C)
60
40
When these two metals are placed in contact, which of the following will take place?
Oa. No heat will flow in either direction.
Ob. Heat will flow from Al to Cu because Al has a larger specific heat.
Oc. Heat will flow from Al to Cu because Al has a larger mass.
Od. Heat will flow from Al to Cu because Al is at a higher temperature.
e. Heat will fiow from Cu to Al because Al has a larger heat capacity.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax