Calculate the concentration of Fe3+ ion in reaction tubes 1-5 of the experiment (after mixing with the SCN-). 1 2 3 4 5 Tube # Show a sample dilution calculation Final [Fe3+]
Calculate the concentration of Fe3+ ion in reaction tubes 1-5 of the experiment (after mixing with the SCN-). 1 2 3 4 5 Tube # Show a sample dilution calculation Final [Fe3+]
Chapter10: Potentiometry And Redox Titrations
Section: Chapter Questions
Problem 8P
Related questions
Question
HANDWRITTEN ONLY... do it as quick as possible....
![Calculate the concentration of Fe3+ ion in reaction tubes 1-5 of the experiment (after mixing with the
SCN-).
1
2
3
4
5
Tube
#
Show a sample dilution calculation
Final [Fe3+]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F2a827fec-b7c2-45ac-b746-52696cce0f4a%2Fffe9f038-cc8d-4c4a-a5fc-8820bc2eda02%2Fhf6cxif_processed.png&w=3840&q=75)
Transcribed Image Text:Calculate the concentration of Fe3+ ion in reaction tubes 1-5 of the experiment (after mixing with the
SCN-).
1
2
3
4
5
Tube
#
Show a sample dilution calculation
Final [Fe3+]
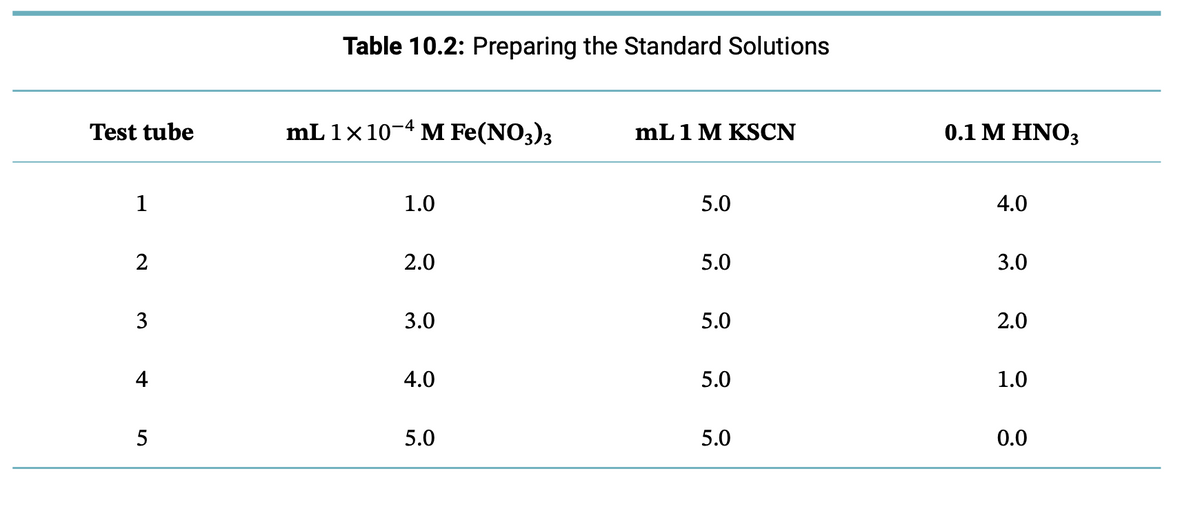
Transcribed Image Text:Test tube
1
2
3
4
5
Table 10.2: Preparing the Standard Solutions
mL 1 x 10-4 M Fe(NO3)3
1.0
2.0
3.0
4.0
5.0
mL1M KSCN
5.0
5.0
5.0
5.0
5.0
0.1 M HNO3
4.0
3.0
2.0
1.0
0.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



