Calculate the density of NaCl given it has a rock salt crystal structure. Na has an atomic weight of 22.99 g/mol, Cl has an atomic weight of 35.45 g/mol. Na has a radii of 0.102nm and CI has a radii of 0.181nm. Answer:
Calculate the density of NaCl given it has a rock salt crystal structure. Na has an atomic weight of 22.99 g/mol, Cl has an atomic weight of 35.45 g/mol. Na has a radii of 0.102nm and CI has a radii of 0.181nm. Answer:
Understanding Motor Controls
4th Edition
ISBN:9781337798686
Author:Stephen L. Herman
Publisher:Stephen L. Herman
Chapter44: Semiconductors
Section: Chapter Questions
Problem 4RQ
Related questions
Question
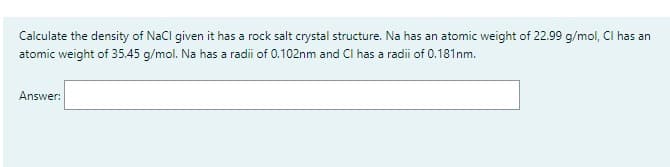
Transcribed Image Text:Calculate the density of NaCl given it has a rock salt crystal structure. Na has an atomic weight of 22.99 g/mol, Cl has an
atomic weight of 35.45 g/mol. Na has a radii of 0.102nm and CI has a radii of 0.181nm.
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Understanding Motor Controls
Mechanical Engineering
ISBN:
9781337798686
Author:
Stephen L. Herman
Publisher:
Delmar Cengage Learning

Welding: Principles and Applications (MindTap Cou…
Mechanical Engineering
ISBN:
9781305494695
Author:
Larry Jeffus
Publisher:
Cengage Learning

Understanding Motor Controls
Mechanical Engineering
ISBN:
9781337798686
Author:
Stephen L. Herman
Publisher:
Delmar Cengage Learning

Welding: Principles and Applications (MindTap Cou…
Mechanical Engineering
ISBN:
9781305494695
Author:
Larry Jeffus
Publisher:
Cengage Learning