Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter11: Quantum Mechanics: Model Systems And The Hydrogen Atom
Section: Chapter Questions
Problem 11.61E: What is the physical explanation of the difference between a particle having the 3-D rotational...
Related questions
Question
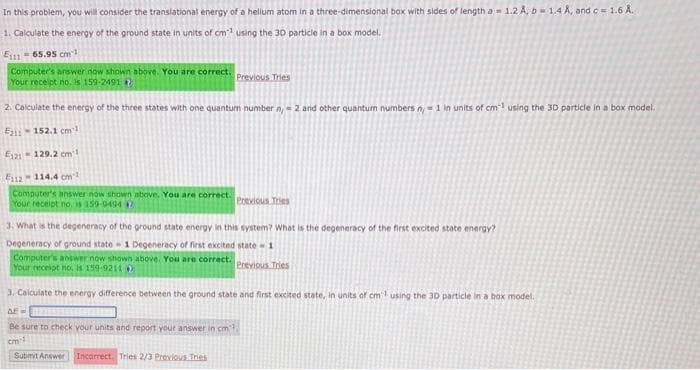
Transcribed Image Text:In this problem, you will consider the translational energy of a helium atom in a three-dimensional box with sides of length a 1.2 A, b= 1.4 A, and c = 1.6 A.
1. Calculate the energy of the ground state in units of cm¹ using the 30 particle in a box model.
E11165.95 cm
Computer's answer now shown above. You are correct.
Your receipt no. is 159-2491
2. Calculate the energy of the three states with one quantum number n = 2 and other quantum numbers n, 1 in units of cm3 using the 3D particle in a box model.
E11-152.1 cm3
E121 129.2 cm²
F112-114.4 cm²
Computer's answer now shown above. You are correct.
Your receipt no. 159-9494
Previous Tries
3. What is the degeneracy of the ground state energy in this system? What is the degeneracy of the first excited state energy?
Degeneracy of ground state 1 Degeneracy of first excited state-1
Computer's answer now shown above. You are correct.
Your receipt no. is 159-9211
Previous Tries
Submit Answer
Previous Tries
3. Calculate the energy difference between the ground state and first excited state, in units of cm using the 30 particle in a box model.
AF=
Be sure to check your units and report your answer in cm3,
cm 1
Incorrect. Tries 2/3 Previous Tries
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,