Calculate the enthalpy change (in kJ) associated with the conversion of 25.0 grams of ice at -4.00 °C to water vapor at 109.0 °C. The specific heats of ice, water, and steam are 2.09 J/g°C, 4.18 J/g°C, and 1.84 J/g°C, respectively. For H,O, AH, = 6.01 kJ/mol and AH = 40.67 kJ/mol.
Calculate the enthalpy change (in kJ) associated with the conversion of 25.0 grams of ice at -4.00 °C to water vapor at 109.0 °C. The specific heats of ice, water, and steam are 2.09 J/g°C, 4.18 J/g°C, and 1.84 J/g°C, respectively. For H,O, AH, = 6.01 kJ/mol and AH = 40.67 kJ/mol.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter14: Liquids And Solids
Section: Chapter Questions
Problem 4QAP: he enthalpy (H)of vaporization of water is about seven times larger than water’s enthalpy...
Related questions
Question
Transcribed Image Text:8:11 PM Tue Sep 14
O 22% O
AA
A us.bbcollab.com
> Chapter 12 - Bb Collaborate
Bb Achieve Assignments - 202130 Fall CHEM-161-BXA (CRN-...
Heating Curve Practice
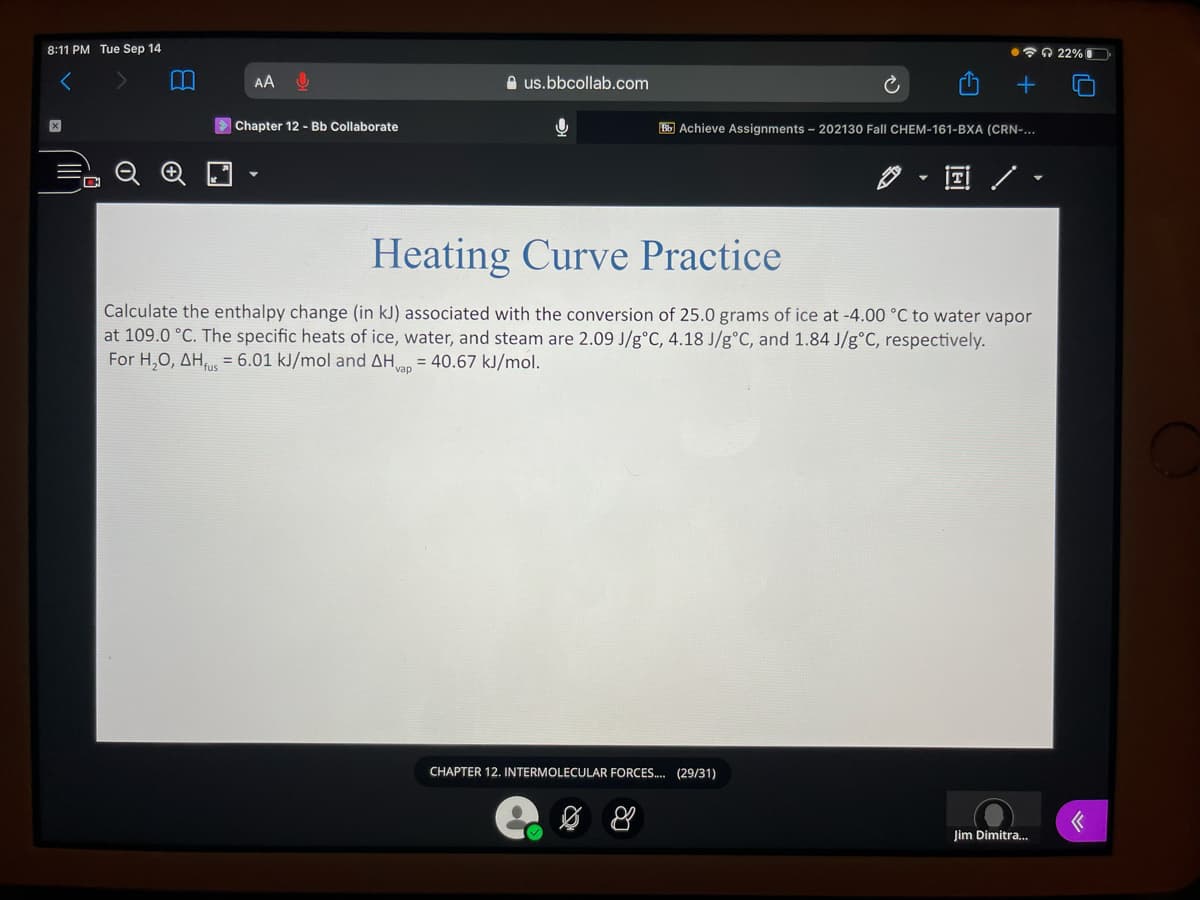
Calculate the enthalpy change (in kJ) associated with the conversion of 25.0 grams of ice at -4.00 °C to water vapor
at 109.0 °C. The specific heats of ice, water, and steam are 2.09 J/g°C, 4.18 J/g°C, and 1.84 J/g°C, respectively.
For H,O, AH, = 6.01 kJ/mol and AH = 40.67 kJ/mol.
CHAPTER 12. INTERMOLECULAR FORCES. (29/31)
Jim Dimitra...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning