Two 20.0 g ice cubes at –14.0 °C are placed into 275 g of water at 25.0 °C. Assuming no energy is transferred to or from the surroundings, calculate the final temperature, T;, of the water after all the ice melts. heat capacity of H,0(s) heat capacity of H,O(1) enthalpy of fusion of H,O 37.7 J/(mol-K) 75.3 J/(mol-K) 6.01 kJ/mol
Two 20.0 g ice cubes at –14.0 °C are placed into 275 g of water at 25.0 °C. Assuming no energy is transferred to or from the surroundings, calculate the final temperature, T;, of the water after all the ice melts. heat capacity of H,0(s) heat capacity of H,O(1) enthalpy of fusion of H,O 37.7 J/(mol-K) 75.3 J/(mol-K) 6.01 kJ/mol
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 102E: A 0.250-g chunk of sodium metal is cautiously dropped into a mixture of 50.0 g water and 50.0 g ice,...
Related questions
Question
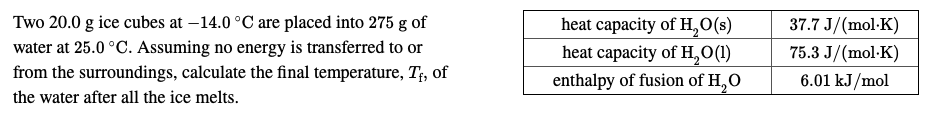
Transcribed Image Text:Two 20.0 g ice cubes at –14.0 °C are placed into 275 g of
water at 25.0 °C. Assuming no energy is transferred to or
from the surroundings, calculate the final temperature, T;, of
the water after all the ice melts.
heat capacity of H,0(s)
heat capacity of H,O(1)
enthalpy of fusion of H,O
37.7 J/(mol-K)
75.3 J/(mol-K)
6.01 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning