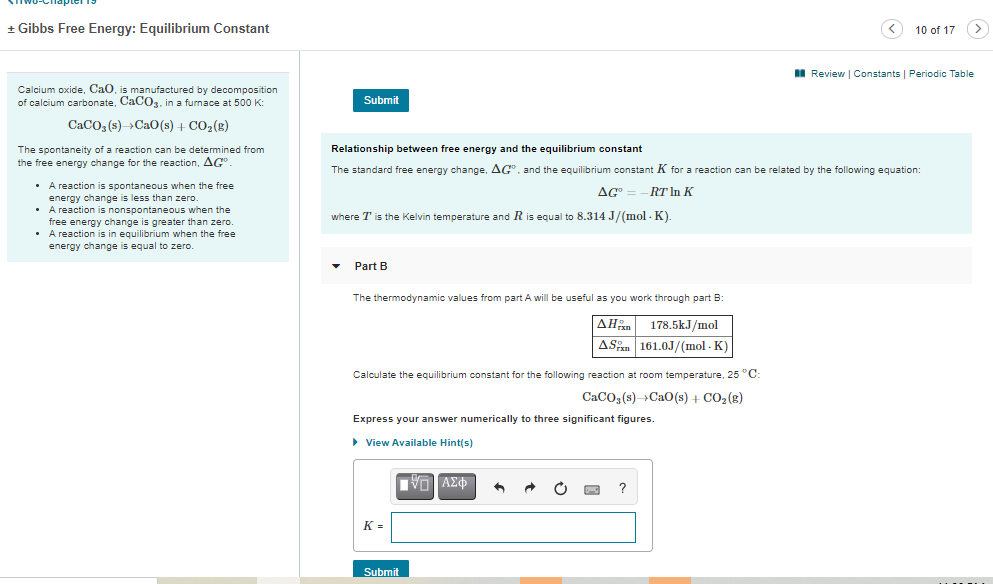
Calculate the equilibrium constant for the following reaction at room temperature, 25 ∘C∘C: CaCO3(s)→CaO(s)+CO2(g)CaCO3(s)→CaO(s)+CO2(g) Express your answer numerically to three significant figures.
Calculate the equilibrium constant for the following reaction at room temperature, 25 ∘C∘C: CaCO3(s)→CaO(s)+CO2(g)CaCO3(s)→CaO(s)+CO2(g) Express your answer numerically to three significant figures.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter16: Thermodynamics
Section: Chapter Questions
Problem 61E: The evaporation of one mole of water at 298 K has a standard free allergy change of 8.58 kJ....
Related questions
Question
Calculate the equilibrium constant for the following reaction at room temperature, 25 ∘C∘C:
CaCO3(s)→CaO(s)+CO2(g)CaCO3(s)→CaO(s)+CO2(g)
Express your answer numerically to three significant figures.
Transcribed Image Text:+ Gibbs Free Energy: Equilibrium Constant
10 of 17
I Review | Constants | Periodic Table
Calcium oxide, CaO, is manufactured by decomposition
of calcium carbonate, CaCO3. in a furnace at 500 K:
Submit
CaCO3 (s)Ca0(s) + CO2(g)
The spontaneity of a reaction can be determined from
Relationship between free energy and the equilibrium constant
the free energy change for the reaction, AG
The standard free energy change, AG". and the equilibrium constant K for a reaction can be related by the following equation:
• A reaction is spontaneous when the free
AG
RT In K
energy change is less than zero.
A reaction is nonspontaneous when the
free energy change is greater than zero.
• A reaction is in equilibrium when the free
where T is the Kelvin temperature and R is equal to 8.314 J/(mol - K).
energy change is equal to zero.
Part B
The thermodynamic values from part A will be useful as you work through part B:
| ΔΗ
ASan 161.0J/(mol - K)
178.5kJ/mol
Calculate the equilibrium constant for the following reaction at room temperature, 25 °C:
CaCO; (s)Cao(s) + CO2 (8)
Express your answer numerically to three significant figures.
> View Available Hint(s)
Πναι ΑΣφ
K =
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning