Calculate the free-energy difference between the stable and unstable chair conformation of each the following trimethylhexanes. CH, CH, CH3 CH3 "CH3 B Support your answer with the drawing of conformer(s) and show your calculations. Energy cost by: H+CH3 eclipsed CH;++CH, gauche 1,3-diaxial (He»CH;) strain 1,3-diaxial (CH3+ACH;) strain Energy (kJ mol"') 6.0 3.8 3.8 15.4
Calculate the free-energy difference between the stable and unstable chair conformation of each the following trimethylhexanes. CH, CH, CH3 CH3 "CH3 B Support your answer with the drawing of conformer(s) and show your calculations. Energy cost by: H+CH3 eclipsed CH;++CH, gauche 1,3-diaxial (He»CH;) strain 1,3-diaxial (CH3+ACH;) strain Energy (kJ mol"') 6.0 3.8 3.8 15.4
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter14: Elimination
Section: Chapter Questions
Problem 30E
Related questions
Question
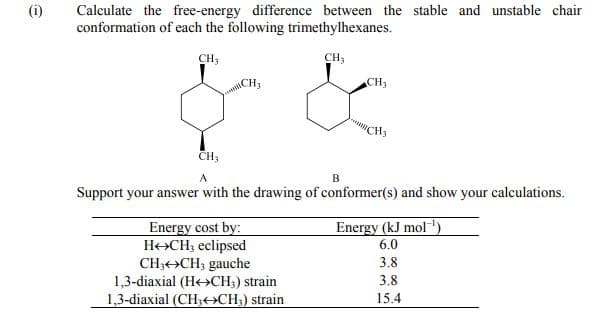
Transcribed Image Text:Calculate the free-energy difference between the stable and unstable chair
conformation of each the following trimethylhexanes.
(i)
CH3
CH;
CH3
CH3
CH,
A
Support your answer with the drawing of conformer(s) and show your calculations.
Energy (kJ mol')
Energy cost by:
H+CH; eclipsed
CH3+>CH; gauche
1,3-diaxial (He>CH3) strain
1,3-diaxial (CH3<CH;) strain
6.0
3.8
3.8
15.4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 5 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
