Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 10CR
Related questions
Question
Calculate the freezing point of this solution
Calculate the partial pressure of chloroform in this solution
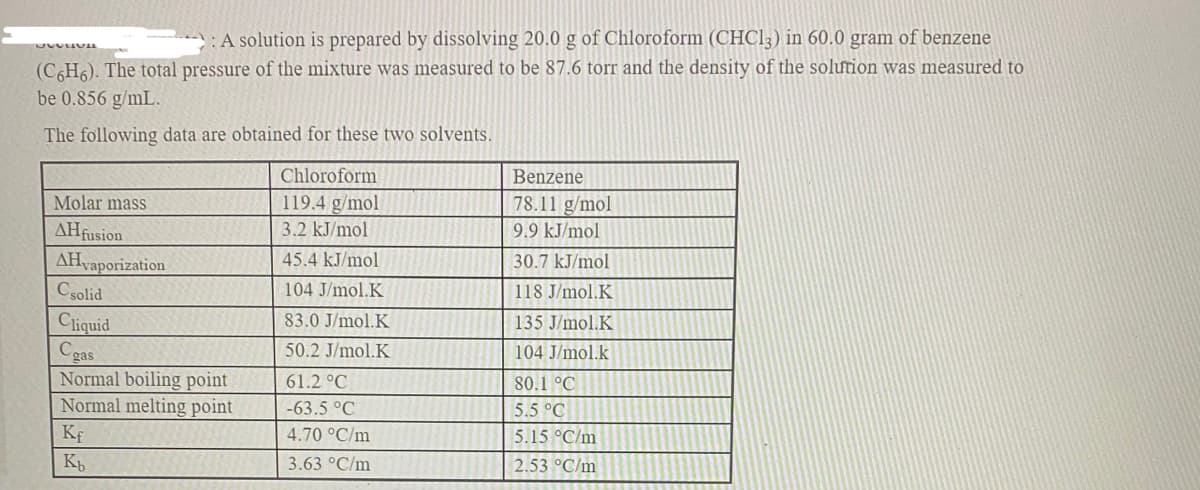
Transcribed Image Text:: A solution is prepared by dissolving 20.0 g of Chloroform (CHC13) in 60.0 gram of benzene
(C6H6). The total pressure of the mixture was measured to be 87.6 torr and the density of the solution was measured to
be 0.856 g/mL.
The following data are obtained for these two solvents.
Chloroform
Benzene
Molar mass
119.4 g/mol
78.11 g/mol
AHfusion
3.2 kJ/mol
9.9 kJ/mol
AHvaporization
45.4 kJ/mol
30.7 kJ/mol
Csolid
104 J/mol.K
118 J/mol.K
Cliquid
Cgas
Normal boiling point
Normal melting point
83.0 J/mol.K
135 J/mol.K
50.2 J/mol.K
104 J/mol.k
61.2 °C
80.1 °C
-63.5 °C
5.5 °C
Kf
4.70 °C/m
5.15 °C/m
K
3.63 °C/m
2.53 °C/m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning