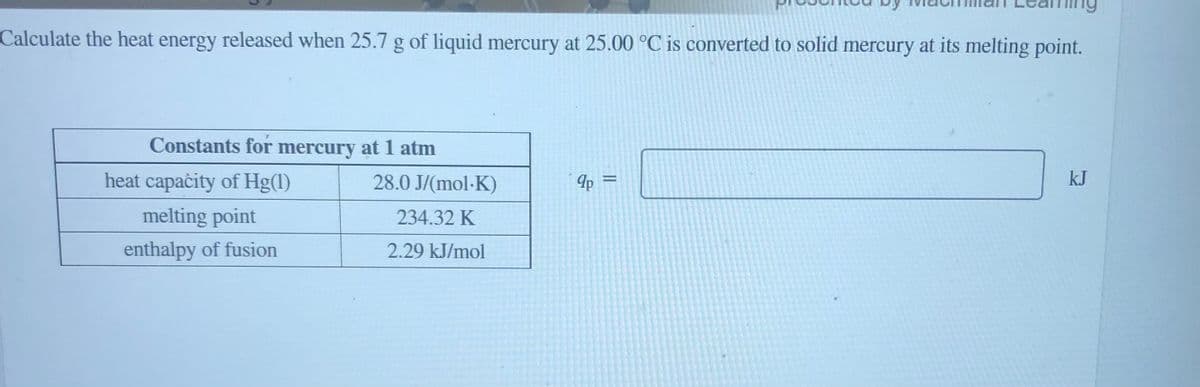
Calculate the heat energy released when 25.7 g of liquid mercury at 25.00 °C is converted to solid mercury at its melting point. Constants for mercury at 1 atm heat capaċity of Hg(1) 28.0 J/(mol-K) 4p = kJ melting point 234.32 K enthalpy of fusion 2.29 kJ/mol
Q: Use the Michaelis-Menten equation to determine the velocity of reaction when: • [S] = 15.0 mM Vmax =…
A: Michaelis-Menten equation is the important equation that deals with enzyme kinetics. The reactants…
Q: Calculate the Keq (report up to two decimal places and do not use scientific notation) for the net…
A: The energetically unfavorable reactions having a positive delta G value is made possible ein the…
Q: If Km = 2.8 µM and concentration of S = 1.36 µM, the velocity of the reaction will be: Give your…
A: Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: If there is 10 μmol of the radioactive isotope 32P (half-life 14 days) at t = 0, how much 32P will…
A: In the process of radioactive decay, a radioactive unstable nucleus loses its energy by radiation.…
Q: What factors determine the relative intensity of transitions in a pure rotational absorption…
A: Absorption spectroscopy is a spectroscopic technique that is based on the absorption of radiation by…
Q: The following reaction plays a key role in the destruction of ozone in the atmosphere: Cl(g)+ O3…
A: Entropy is a measure of the randomness of a system. It the measure of a system's energy per unit…
Q: The Gibbs free energy can be defined as the maximum amount of non-expansion work performed by a…
A:
Q: How many kilojoules are released by freezing 2.5 mol of H2O?
A: Enthalpy of freezing (∆Hfree) is used to calculate energy needed to release when liquid becomes…
Q: The energy of activation for the reaction 2 HI - H2 + I2 is 180. kJ-mol-1 at 544 K. Calculate the…
A:
Q: Enzyme A, B, and C having the following properties are to be separated by various chromatography at…
A: The chromatographic process is generally helping a solvent that destroys an adsorbed substance from…
Q: What is the total entropy change if a 0.310 efficiency engine takes 3.96E3 J of heat from a 304 degC…
A: Given the δQH of energy flow into the system the system's entropy changes by: dSsys=δQH/Tsys
Q: In some cases, the Km and Vmax can be estimated by inspecting the values of the initial velocity (V…
A: Enzymes are proteins that catalyze biochemical reactions. They are classified into six classes-…
Q: For a Michaelis Menten reaction the following rate constants were observed: k1 =7x 10/M-sec k1=1x…
A: The correct answer is option, d. 0.0003M.
Q: Given the following values for the changes in enthalpy (AH) and entropy (AS), which of the following…
A: Introduction: The correct choice is option (a) and (b).
Q: For the series of reactions below, what is the overall reaction and the value of ∆Go’(kJ/mol) for…
A: Consider a set of reactions 1 and 2; Reaction 1 : a→b have a ∆G0' = y Reaction 2 : c→d have a ∆G0' =…
Q: For the following reaction 3 experiments have been run and the data collected is in the following…
A: a)
Q: Sugar solution is being heated to 83 o C in a jacketed pan made from stainless steel, 1.6 mm thick.…
A: The heat flow or work indicates the energy transfer on or by the system in chemical thermodynamic.…
Q: What information can be obtained from a kinetic data where 1/v is equal to 0.5 min/mole, when 1/[S]…
A:
Q: The temperature dependence of the vapor pressure is given by the equation: Inp = In A – RT Where: p=…
A: Thermodynamics is a study that deals with the relationship between work, heat, temperature, and…
Q: Consider a reaction with ΔH = 15 kJ and ΔS = 50 J · K−1. Is the reaction spontaneous (a) at 10°C,…
A: Gibbs free energy refers to the chemical energy associated with the reaction that is used to do…
Q: 2. For each of the following gas-phase reactions, write the rate expression in terms of the…
A: The rate of reaction is the transformation of a reactant or a product per unit of time. Due to this,…
Q: Solve this problem
A: Given, The amount of heat absorbed is 26.06 J. The mass of the diamond is 2.10 g. The initial…
Q: Chloroform is a volatile (readily changes from liquid phase to gaseous phase) once commonly used in…
A: Chloroform is an organic compound which is colorless, strong-smelling, dense liquid with a formula…
Q: (a) How much time is needed to measure the kinetic energy of an electron whose speed is 10.0 m/s…
A: We are answering part a only pls repost for part b
Q: Answer the following questions yes or no (Y/N): Is the michaelis-menten equation valid under…
A: Note: Hi ! Thank you for the question. We are authorized to answer three subparts at a time. Since…
Q: Under what conditions is the entropy H(X) equal to zero?
A: Gases expand on heating. Heat energy enhances the vibrational displacement of gaseous molecules.…
Q: Which has greater entropy, liquid water at 0°C or ice at 0°C?
A: The measurement of the thermal energy of the particular structure with temperature when there is no…
Q: Consider the following process: NaCl(s) water−→−−water→ Na+(aq) + Cl-(aq); ΔH = +4.2 kJ/mol Under…
A: ΔG stands for gibbs free energy and determine the favourability of a reaction. It depend on various…
Q: Calculate the equilibrium constant (K'eq) for each of the three reactions at pH 7.0 and 25 °C, using…
A:
Q: Using the following plot, calculate the change in entropy (J/mol K). 2.000 In(K,) vs 1/T(K) 1.500…
A: In the field of chemical kinetics, Arrhenius plot gives the log of a reaction rate constant ln (k),…
Q: The formation constants at 25°C for Fe(CN)4-6 and Fe(EDTA)2– are 1.00 x 1037 and 2.10 x 1014,…
A: Gibbs free energy is the amount of energy that is used to calculate the work done by a system under…
Q: define the equilibrium constant KP for the chemical equilibrium ofideal-gas mixtures?
A: Kp is the equilibrium constant given by the partial pressures of a reaction. This gas constant is…
Q: Question attached
A: The photons are referred to as the elementary particles that have the role of serving as a quantum…
Q: Write the nuclear equation for the alpha decay of Po-214.
A: The radioactive decay process is defined as the loss of energy by an unstable nucleus via emitting…
Q: Write the nuclear equation for the positron decay of C-11.
A: C-11 is a radioisotope of carbon. C-11 is an unstable atom that disintegrates by emitting radiation.…
Q: (a) (' . Hill plots. Label the axes and indicate on both the plot above and the Hill plot where the…
A: We know that nitrous oxide are anesthetic gas that are used during surgery and are non volatile in…
Q: SITUATION: You are a chemist in a manufacturing company which produces acetaldehyde, CH3CHO. This is…
A: Introduction CH3CH2OH+CuO→CH3CHO+H2O+Cu 1 mole 1 mole 1 moleMolar mass…
Q: the activation energy for the reaction described below is kJ. [to answer, you must identify two…
A: Activation energy is the minimum amount of energy required to perform a reaction.
Q: If experimental determinations of Kp at different temperatures for a given equilibrium in the…
A: Kp is the equilibrium constant calculated from partial pressures of reaction equation. It is a…
Q: hydrogen at 101 kpa each day . If each days supply of hydrogen were kept at a pressure of 366 kpa,…
A: The relationship for Boyle's Law can be expressed as follows: P1V1 = P2V2, where P1 and V1 are the…
Q: A helium gas cylinder of the sort used to fill balloons has a volume of 0.180 m3 and a pressure of…
A: The ideal gas law is equation of state of a hypothetical ideal gas where the Pressure, Volume,…
Q: Calculate the standard Gibbs energy of formation of H,O(g) at 25 °C.
A: The reaction of the formation of water is: 2H2(g)+O2(g)⟶2H2O(l) Gibb's Free Energy calculation…
Q: How many kilojoules are released by the combustion of 17.0 g of C8H18?
A: Carbon is the key element fount in all organic molecules. carbon have 4 valency. Based on the carbon…
Q: How is Kw defined, and what is its numerical value at 25 °C (298 K)?
A: As we know that the pure water is a considered as weak electrolyte that is H2O ⇔H++ OH-
Q: A reaction at 23°C has ∆G = 1 kJ mol–¹. Why might this reaction become spontaneous at 37°C?
A: A reaction at 23 degree celecius has 1 KJ mol-1 This would make the G value negative and hence the…
Q: in the noncompetitive inhibtion If Km = 2 mM, and Vo = 100 umol.s when [S] = 2 mM, what is the…
A: Introduction: Those substances that decrease the catalytic activity of the enzyme during the…
Q: Calculate the work done by n moles of a van der Waals gas in an isothermal expansion (at constant…
A: Van der Waals equation is defined an equation of the state, in thermodynamics that generalizes the…
Q: The amount of heat in kJ required to convert 0.7 gal of liquid water to steam equal… ?
A: 0.7 gallon of water 1 gallon = 3.7854 kg water 0.7 gallon = 2.65 kg water
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

- The standard heat of combustion of liquid methyl cyclopentane, C6H12(l),C6H12(l), was measured to be −3937.7 kJ/mol.−3937.7 kJ/mol. What is Δ?̂ ∘f C6H12(l),ΔH^f C6H12(l)∘, the standard heat of formation of liquid methyl cyclopentane?How is Kw defined, and what is its numerical value at 25 °C (298 K)?For ferrocene (C10H10Fe) the enthalpy of sublimation is 73.2 kJ/mol and the entropy of sublimation is 243 J/mol.K. What is the sublimation temperature of ferrocene in degrees Celsius?
- The amount of heat in kJ required to convert 0.7 gal of liquid water to steam equal… ?The following reaction plays a key role in the destruction of ozone in the atmosphere: Cl(g)+ O3 (g)-> ClO(g)+O2 (g) Given the standard molar entropies (S°) below, calculate the ΔS for this reaction. S°C1O = 218.9 J/mol*K S°O3 = 238.8 J/mol*K S°Cl = 165.2 J/mol*K S°O2= 205.0 J/mol*K _______ J/K = ΔSHow many kilojoules are released by freezing 2.5 mol of H2O?
- Calculate the Keq (report up to two decimal places and do not use scientific notation) for the net reaction at 298.15K. (see attached image) Note: R = 1.98 x 10 -3 kcal/mol-KCalculate ΔG° (answer in kJ/mol) for each of the following reactions from the equilibrium constant at the temperature given. (d)CoO(s)+CO(g)⇌Co(s)+CO2(g) T=550°C Kp=4.90×102 (e)CH3NH2(aq)+H2O(l)⟶CH3NH3+(aq)+OH−(aq) T=25°C Kp=4.4×10−4 (f)PbI2(s)⟶Pb2+(aq)+2I−(aq) T=25°C Kp=8.7×10Po-210 is an alpha emitter with a half-life of 138 days. Howmany grams of Po-210 remain after 552 days if the sampleinitially contained 5.80 g of Po-210?
- For the following reaction 3 experiments have been run and the data collected is in the following table @ 35 degrees Celsius 2 NO2F(g) ---> 2 NO2(g) + F2(g) Experiment [NO2F], M Rates, M/s 1 0.263 0.168 2 0.349 0.223 3 0.421 0.269 a) How long will it take for a 65% NO2F solution to become a 31% NO2F solution @35 degrees Celsius?(Hint: Use mass ratios and assume ~1g/ml for density of solutions to get you started) b) It has been determined that at 75 degrees Celsius the rate constant is 1.046 s-1. Calculate the activation energy for the decomposition of NO2F. [Hint: ]ln?1?2=?a?(1?2―1?1) c) What is the half-life of a 35% solution of NO2F @ 35 degrees Celsius?Ethylene oxide is produced industrially from the reaction of ethylene with oxygen at atmospheric pressure and 277 oC, in the presence of silver catalyst.C2H4(g) + O2(g) → C2H4O(g) (unbalanced)Assuming 100 % yield, how many kg of ethylene oxide can be produced from 34600 L of a mixture containing ethylene and oxygen in 1:1 molar ratio?in the noncompetitive inhibtion If Km = 2 mM, and Vo = 100 umol.s when [S] = 2 mM, what is the velocity Vo for the reaction when [S] = 18 mM ? 360 umol.s 180 umol.s 90 umol.s 200 umol.s

