The amount of heat (g) gained or lost by a substance (with mass m) as its temperature changes (AT) depends on its specific heat capacity (C,) according to the following equation. q - C, xm x AT The quality of industrial diamonds is determined in part by measuring the specific heat capacity, which is 0.5091 /g-K for pure diamond. If the absorption of 26.06 ) of heat by a 2.10g diamond sample of unknown purity causes its temperature to rise from 25.90°C to 73.45°C, is the diamond sample pure? Explain your reasoning. The calculated C, is J/g-K and the value for pure diamond is 0.5091 J/g-K. These values are --Select- , thus the sample ---Select- pure. Need Help? Master it A foundry worker places a 5.76 kg sheet of nickel at a temperature of 18°C on top of a 16.6 kg sheet of palladium at 65°C. Assuming no heat is lost to the surroundings, calculate the final temperature of the two shects of metal. °C Need Help?Master It
The amount of heat (g) gained or lost by a substance (with mass m) as its temperature changes (AT) depends on its specific heat capacity (C,) according to the following equation. q - C, xm x AT The quality of industrial diamonds is determined in part by measuring the specific heat capacity, which is 0.5091 /g-K for pure diamond. If the absorption of 26.06 ) of heat by a 2.10g diamond sample of unknown purity causes its temperature to rise from 25.90°C to 73.45°C, is the diamond sample pure? Explain your reasoning. The calculated C, is J/g-K and the value for pure diamond is 0.5091 J/g-K. These values are --Select- , thus the sample ---Select- pure. Need Help? Master it A foundry worker places a 5.76 kg sheet of nickel at a temperature of 18°C on top of a 16.6 kg sheet of palladium at 65°C. Assuming no heat is lost to the surroundings, calculate the final temperature of the two shects of metal. °C Need Help?Master It
Chapter1: Numerals And Fractions
Section: Chapter Questions
Problem 1RP
Related questions
Question
Solve this problem
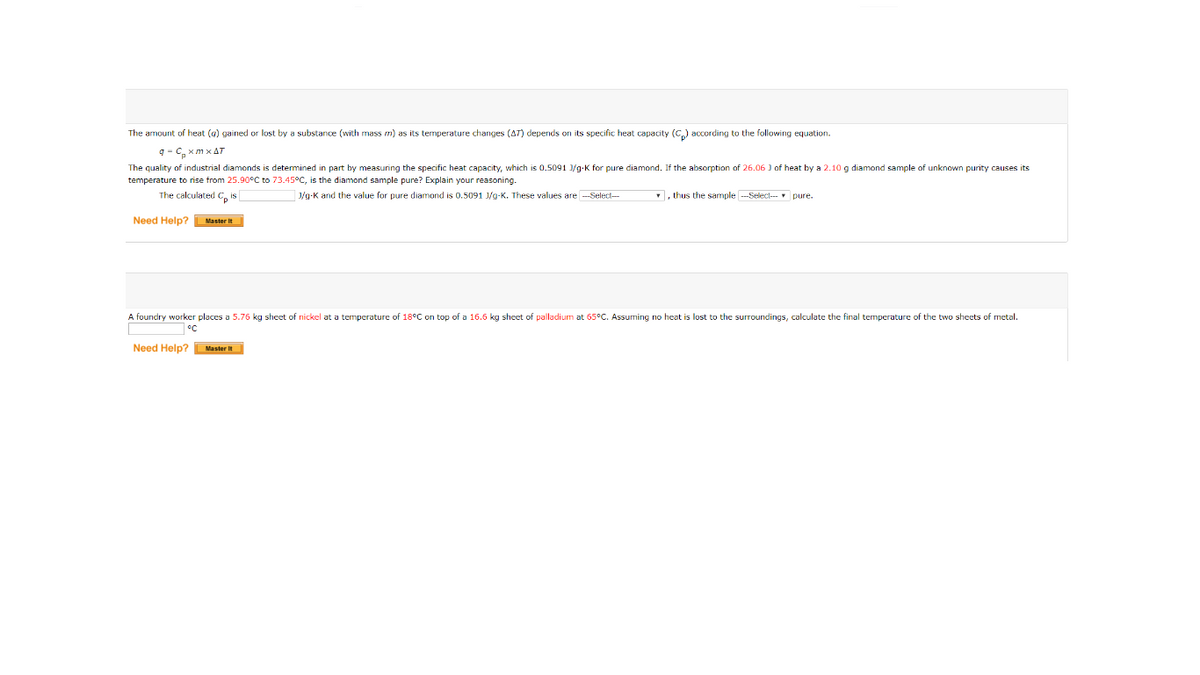
Transcribed Image Text:The amount of heat (g) gained or lost by a substance (with mass m) as its temperature changes (AT) depends on its specific heat capacity (C,) according to the following equation.
q - C, xm x AT
The quality of industrial diamonds is determined in part by measuring the specific heat capacity, which is 0.5091 /g-K for pure diamond. If the absorption of 26.06 ) of heat by a 2.10g diamond sample of unknown purity causes its
temperature to rise from 25.90°C to 73.45°C, is the diamond sample pure? Explain your reasoning.
The calculated C, is
J/g-K and the value for pure diamond is 0.5091 J/g-K. These values are --Select-
, thus the sample ---Select- pure.
Need Help? Master it
A foundry worker places a 5.76 kg sheet of nickel at a temperature of 18°C on top of a 16.6 kg sheet of palladium at 65°C. Assuming no heat is lost to the surroundings, calculate the final temperature of the two shects of metal.
°C
Need Help?Master It
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, nursing and related others by exploring similar questions and additional content below.Recommended textbooks for you



