Calculate the heat of reaction AH for the following reaction: 2 NH3(g) N₂(g) + 3 H₂(g) You can find a table of bond energies by using the Data button on the ALEKS toolbar. Round your answer to the nearest kJ/mol. al Data 0 kJ mol + [-] Bond energies
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
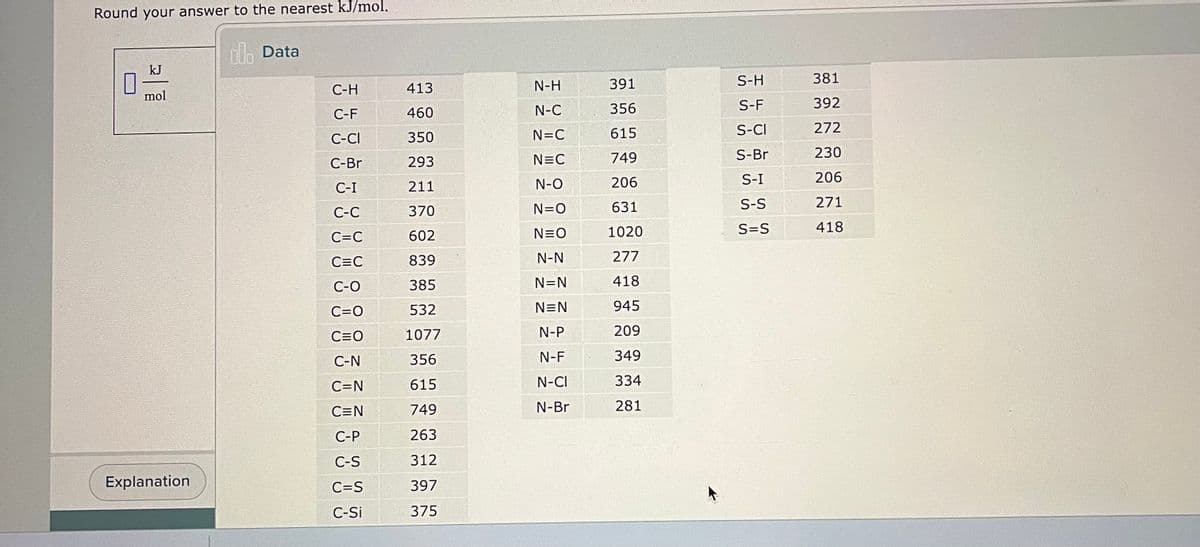
![Calculate the heat of reaction AH for the following reaction:
2 NH3(g) →N₂(g) + 3 H₂(g)
You can find a table of bond energies by using the Data button on the ALEKS toolbar.
Round your answer to the nearest kJ/mol.
0
kJ
mol
Explanation
lo Data
[-] Bond energies
bond
+
H-H
H-F
H-CI
H-Br
H-I
H-C
H-O
H-N
H-P
H-S
energy
(kJ/mol)
436
570
431
366
298
413
497
391
351
381
bond
O-H
O-F
O-CI
O-Br
O-I
O-C
O=C
O=C
O-O
O=O
O-N
O=N
O=S
energy
(kJ/mol)
497
215
234
210
213
385
532
1077
210
498
206
631
551
bond
F-F
F-CI
F-Br
F-I
CI-CI
Cl-Br
CI-I
Br-Br
Br-I
I-I
energy
(kJ/mol)
159
261
280
272
243
219
211
194
179
152](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F4f2989fb-e70f-4c42-ab2b-83db77fa908b%2Fd4aed941-b956-4186-aae0-9cf44e0f1fa3%2Fgw7zw9t_processed.jpeg&w=3840&q=75)
Step by step
Solved in 2 steps with 3 images









