Calculate the lattice enthalpy(nearest integer value) in kJmol-1 of LiF, given that ,enthalpy of sublimation of lithium is 155.3kJ mol-1 (i) ;dissociation of 1/2 mole of F2 is 75.3kJ (ii) ;ionization enthalpy of lithium is 520kJ mol-1 (iii) electron gain enthalpy of 1 mole of F(g) is -333k (iv) AfH overall is -594kJ mol-1 (v)
Calculate the lattice enthalpy(nearest integer value) in kJmol-1 of LiF, given that ,enthalpy of sublimation of lithium is 155.3kJ mol-1 (i) ;dissociation of 1/2 mole of F2 is 75.3kJ (ii) ;ionization enthalpy of lithium is 520kJ mol-1 (iii) electron gain enthalpy of 1 mole of F(g) is -333k (iv) AfH overall is -594kJ mol-1 (v)
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter12: The Solid State
Section12.3: Bonding In Ionic Compounds: Lattice Energy
Problem 3RC
Related questions
Question
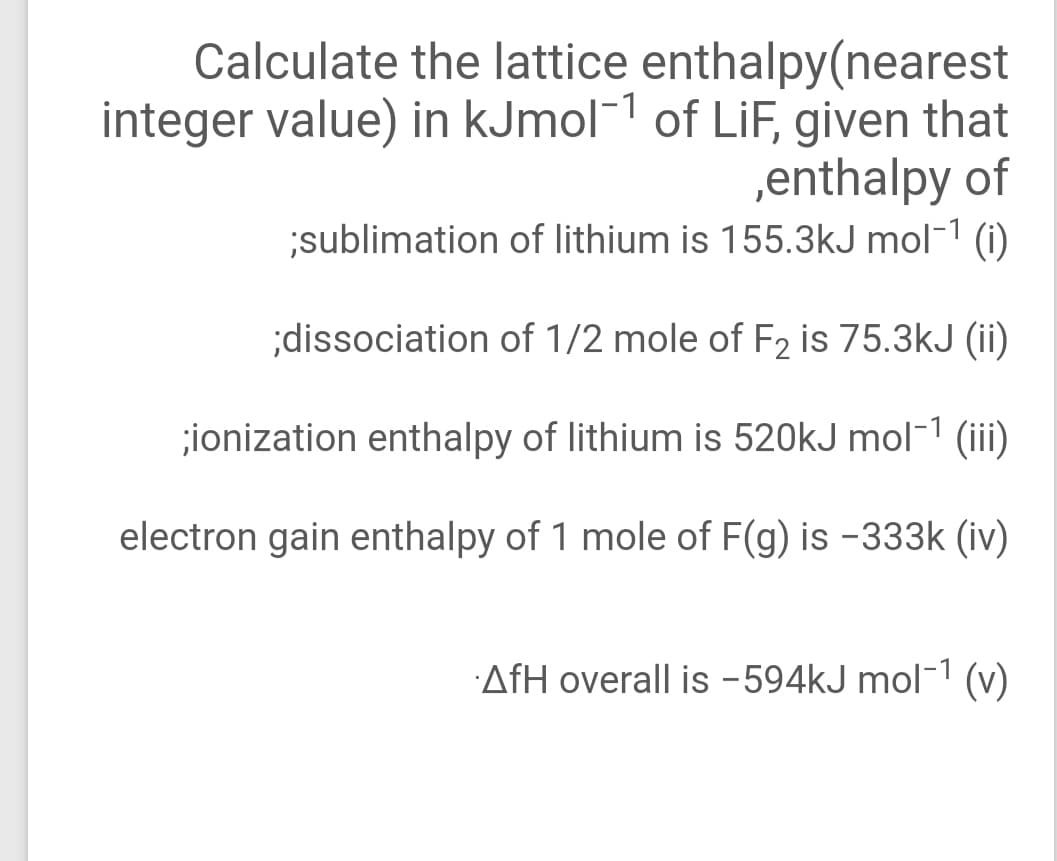
Transcribed Image Text:Calculate the lattice enthalpy(nearest
integer value) in kJmol-1 of LiF, given that
,enthalpy of
sublimation of lithium is 155.3kJ mol-1 (i)
;dissociation of 1/2 mole of F2 is 75.3kJ (ii)
;ionization enthalpy of lithium is 520kJ mol-1 (iii)
electron gain enthalpy of 1 mole of F(g) is -333k (iv)
AfH overall is -594kJ mol-1 (v)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,