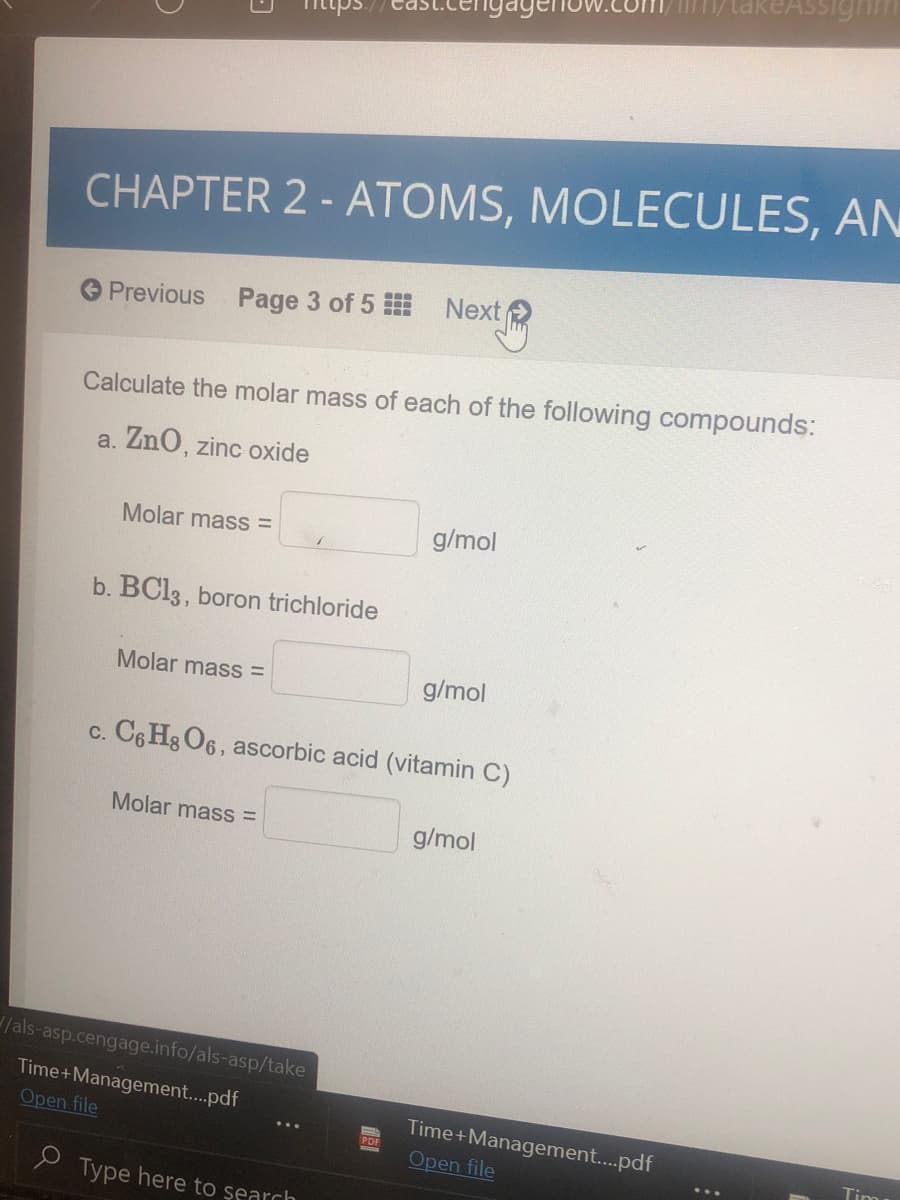
Calculate the molar mass of each of the following compounds: a. ZnO, zinc oxide Molar mass = g/mol b. BC13, boron trichloride Molar mass = g/mol c. C6 Hg O6, ascorbic acid (vitamin C) Molar mass = g/mol
Calculate the molar mass of each of the following compounds: a. ZnO, zinc oxide Molar mass = g/mol b. BC13, boron trichloride Molar mass = g/mol c. C6 Hg O6, ascorbic acid (vitamin C) Molar mass = g/mol
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 38QAP: Calculate the mass in grams of each of the following samples. l type="a"> 0.994 mole of benzene,...
Related questions
Question
Transcribed Image Text:CHAPTER 2 - ATOMS, MOLECULES, AN
Previous Page 3 of 5
Next
Calculate the molar mass of each of the following compounds:
a. ZnO, zinc oxide
Molar mass =
g/mol
b. BC13, boron trichloride
Molar mass =
g/mol
c. C6 Hg O6, ascorbic acid (vitamin C)
Molar mass =
g/mol
/als-asp.cengage.info/als-asp/take
Time+Management..pdf
Time+Management..pdf
Open file
Open file
PDE
Tim
> Type here to şearch
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole