Calculate the molar solubility, S, of Ag2(CrO4) in a 0.138 M solution of K2(CrO,). Note that K2(CrO,) is highly soluble and the K, of Ag2 (CrO,) is 9.00 x 10 12.
Calculate the molar solubility, S, of Ag2(CrO4) in a 0.138 M solution of K2(CrO,). Note that K2(CrO,) is highly soluble and the K, of Ag2 (CrO,) is 9.00 x 10 12.
Chapter9: Aqueous Solutions And Chemical Equilibria
Section: Chapter Questions
Problem 9.8QAP
Related questions
Question
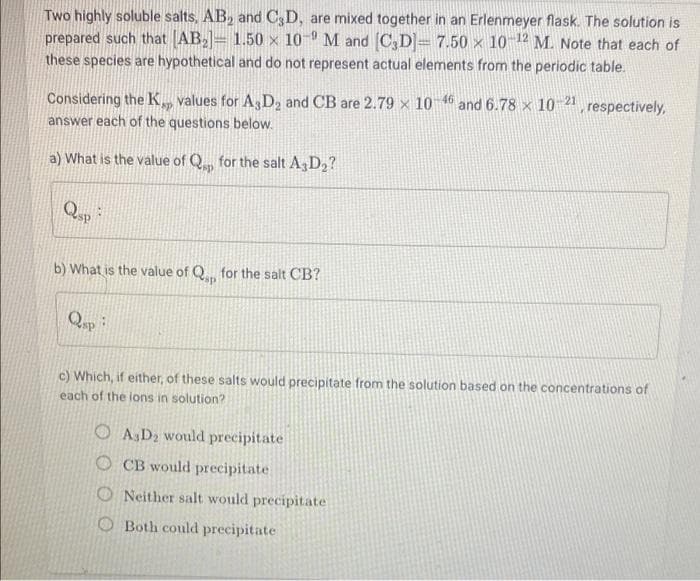
Transcribed Image Text:Two highly soluble salts, AB, and C3D, are mixed together in an Erlenmeyer flask. The solution is
prepared such that AB= 1.50 x 10 M and C,D= 7.50 x 102 M. Note that each of
these species are hypothetical and do not represent actual elements from the periodic table.
Considering the K, values for A3 D2 and CB are 2.79 x 104 and 6.78 x 10 21, respectively,
answer each of the questions below.
a) What is the value of Q, for the salt A3D2?
Qup
b) What is the value of Q. for the salt CB?
c) Which, if either, of these salts would precipitate from the solution based on the concentrations of
each of the lons in solution?
O A,D2 would precipitate
O CB would precipitate
O Neither salt would precipitate
O Both could precipitate

Transcribed Image Text:Calculate the molar solubility, S, of Ag2(CrO,) in a 0.138 M solution of K2(CrO,). Note that
K2(CrO4) is highly soluble and the K of Ag2 (CrO,) is 9.00 x 10-12 .
M
S:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning