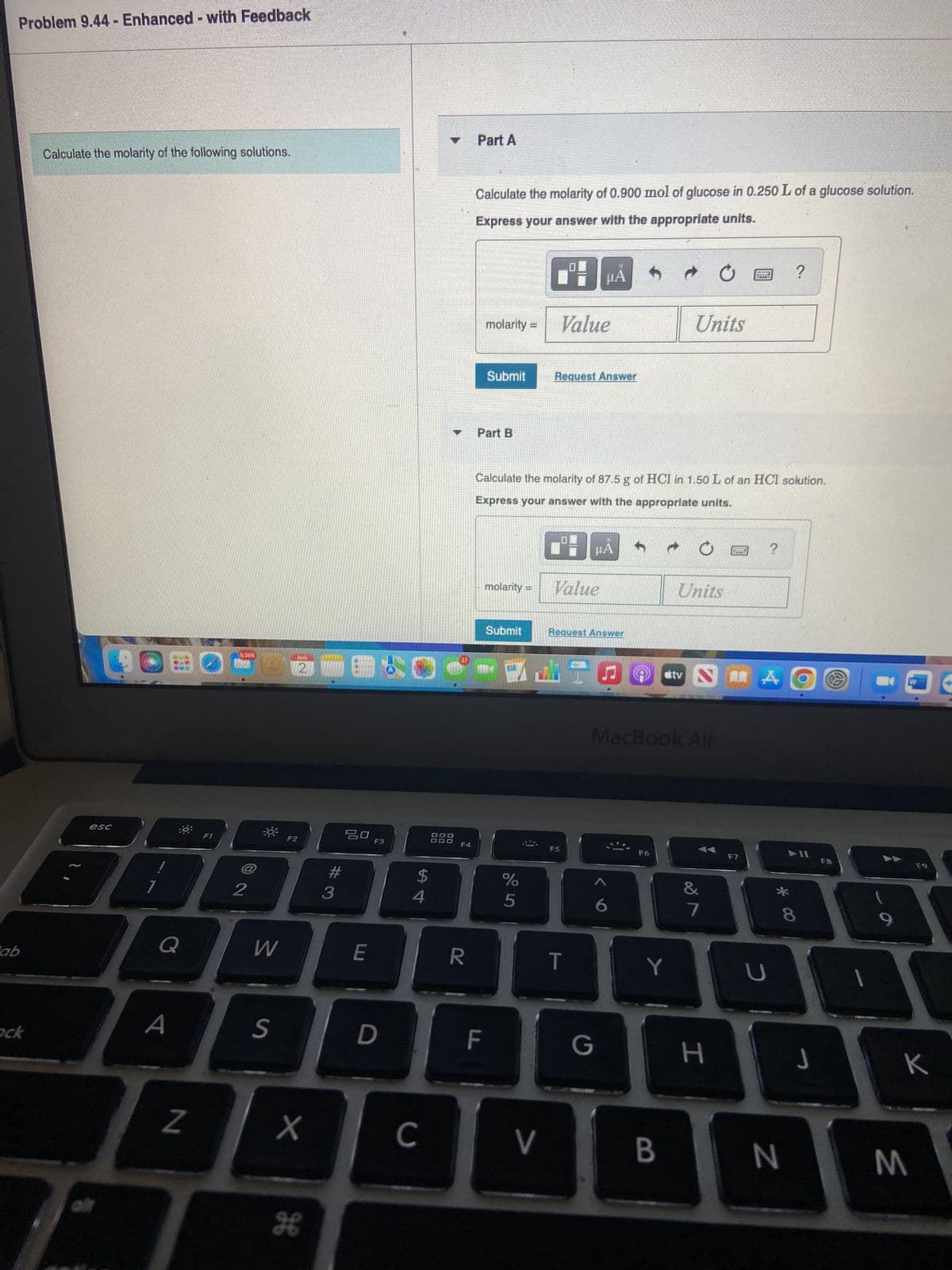
Calculate the molarity of the following solutions. Part A Calculate the molarity of 0.900 mol of glucose in 0.250 L of a glucose soluti Express your answer with the appropriate units. molarity= Value Submit Part B LON molarity = Request Answer HA → Calculate the molarity of 87.5 g of HCI in 1.50 L of an HCl solution. Express your answer with the appropriate units. Value Units Units ? ?
Calculate the molarity of the following solutions. Part A Calculate the molarity of 0.900 mol of glucose in 0.250 L of a glucose soluti Express your answer with the appropriate units. molarity= Value Submit Part B LON molarity = Request Answer HA → Calculate the molarity of 87.5 g of HCI in 1.50 L of an HCl solution. Express your answer with the appropriate units. Value Units Units ? ?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 78QAP: A gaseous solute dissolves in water. The solution process has H=15 kJ. Its solubility at 22C and...
Related questions
Question
Transcribed Image Text:Problem 9.44 - Enhanced - with Feedback
Cab
ock
Calculate the molarity of the following solutions.
esc
Q
A
Z
F1
5,566
2
W
S
AUG
2 E6
F2
N
X
H
#3
20
E
F3
D
$
4
C
888
27
F4
R
Part A
Calculate the molarity of 0.900 mol of glucose in 0.250 L of a glucose solution.
Express your answer with the appropriate units.
molarity =
Submit
Part B
F
molarity
Submit
%
5
V
Calculate the molarity of 87.5 g of HCl in 1.50 L of an HCl solution.
Express your answer with the appropriate units.
Value
Request Answer
μÃ
Value
F5
Request Answer
T
μA
G
F6
MacBook Air
Y
Units
B
Units
tv
&
7
H
7
F7
2
A
?
* 00
N
O
► 11
J
F8
F9
K
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning