Calculate the number of grams of carbon dioxide produced from complete combustion of one liter of octane by placing the conversions in the correct order. Make sure that the units of adjacent conversion factors cancel out. X X 1 LOctaneX 2171 g X Carbon Dioxide 8 mol CO2 114 g Octane 1000 mL Octane 1 mol Octane 1 mol Octane 8 mol CO2 1 mol Octane 1 L Octane 44 g CO2 1 mol Octane 1 mL Octane 0.703 g Octane 114 g Octane 1 mol CO2 0.703 g Octane 1 mL Octane
Calculate the number of grams of carbon dioxide produced from complete combustion of one liter of octane by placing the conversions in the correct order. Make sure that the units of adjacent conversion factors cancel out. X X 1 LOctaneX 2171 g X Carbon Dioxide 8 mol CO2 114 g Octane 1000 mL Octane 1 mol Octane 1 mol Octane 8 mol CO2 1 mol Octane 1 L Octane 44 g CO2 1 mol Octane 1 mL Octane 0.703 g Octane 114 g Octane 1 mol CO2 0.703 g Octane 1 mL Octane
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter6: Chemical Calculations: Formula Masses, Moles, And Chemical Equations
Section: Chapter Questions
Problem 6.68EP: How many carbon monoxide molecules (CO) are needed to react with 8 hydrogen molecules (H2) to...
Related questions
Question
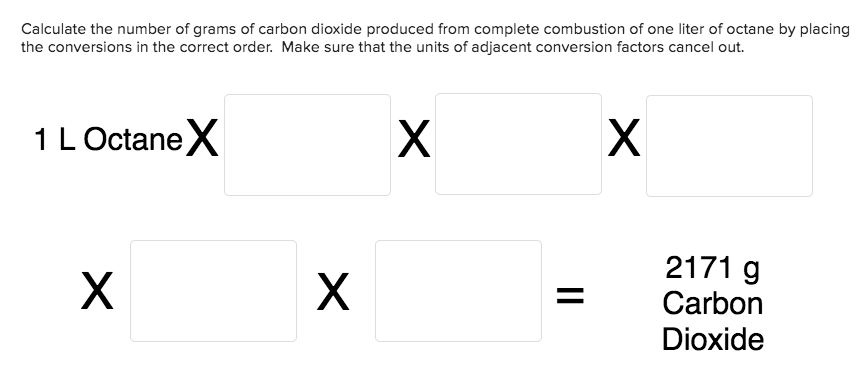
Transcribed Image Text:Calculate the number of grams of carbon dioxide produced from complete combustion of one liter of octane by placing
the conversions in the correct order. Make sure that the units of adjacent conversion factors cancel out.
X
X
1 LOctaneX
2171 g
X
Carbon
Dioxide
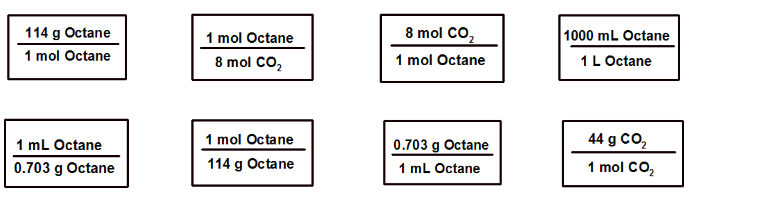
Transcribed Image Text:8 mol CO2
114 g Octane
1000 mL Octane
1 mol Octane
1 mol Octane
8 mol CO2
1 mol Octane
1 L Octane
44 g CO2
1 mol Octane
1 mL Octane
0.703 g Octane
114 g Octane
1 mol CO2
0.703 g Octane
1 mL Octane
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER