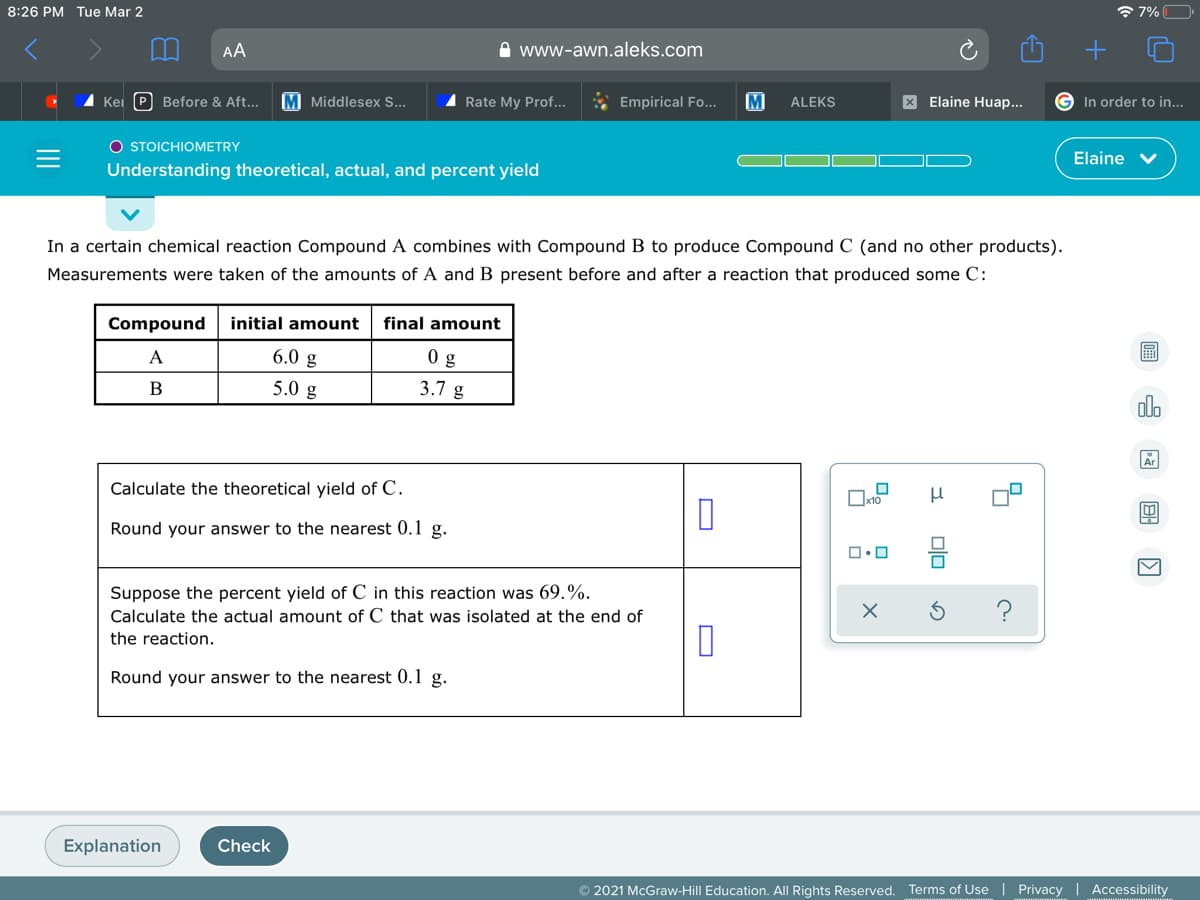
In a certain chemical reaction Compound A combines with Compound B to produce Compound C (and no other products). Measurements were taken of the amounts of A and B present before and after a reaction that produced some C: Compound initial amount final amount A 6.0 g 0 g 5.0 g 3.7 g alo B Calculate the theoretical yield of C. Round your answer to the nearest 0.1 g. Suppose the percent yield of C in this reaction was 69.%. Calculate the actual amount of C that was isolated at the end of ? the reaction. Round your answer to the nearest 0.1 g. 回 国国回
In a certain chemical reaction Compound A combines with Compound B to produce Compound C (and no other products). Measurements were taken of the amounts of A and B present before and after a reaction that produced some C: Compound initial amount final amount A 6.0 g 0 g 5.0 g 3.7 g alo B Calculate the theoretical yield of C. Round your answer to the nearest 0.1 g. Suppose the percent yield of C in this reaction was 69.%. Calculate the actual amount of C that was isolated at the end of ? the reaction. Round your answer to the nearest 0.1 g. 回 国国回
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.14QAP
Related questions
Question
Transcribed Image Text:8:26 PM Tue Mar 2
* 7%O
AA
O www-awn.aleks.com
Kei (P Before & Aft...
M Middlesex S...
I Rate My Prof...
* Empirical Fo..
ALEKS
X Elaine Huap...
In order to in...
O STOICHIOMETRY
Elaine v
Understanding theoretical, actual, and percent yield
In a certain chemical reaction Compound A combines with Compound B to produce Compound C (and no other products).
Measurements were taken of the amounts of A and B present before and after a reaction that produced some C:
Compound
initial amount
final amount
A
6.0 g
0 g
圖
B
5.0 g
3.7 g
do
Ar
Calculate the theoretical yield of C.
Round your answer to the nearest 0.1 g.
Suppose the percent yield of C in this reaction was 69.%.
Calculate the actual amount of C that was isolated at the end of
the reaction.
Round your answer to the nearest 0.1 g.
Explanation
Check
© 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy | Accessibility
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co