5) For the following reaction: L (g) + H. (g) → 2HI (g) If the amount consumed from (I) according to the above reaction is (50.8g) and the actual yield of HI is (40g) then the percentage yield of (HI) is equal to : a) 78.125% b) 15.625% c) 63.341% d)39.06% .
5) For the following reaction: L (g) + H. (g) → 2HI (g) If the amount consumed from (I) according to the above reaction is (50.8g) and the actual yield of HI is (40g) then the percentage yield of (HI) is equal to : a) 78.125% b) 15.625% c) 63.341% d)39.06% .
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter11: Stoichiometry
Section11.4: Percent Yield
Problem 29PP
Related questions
Question
Transcribed Image Text:Feading
seds
Paragraph
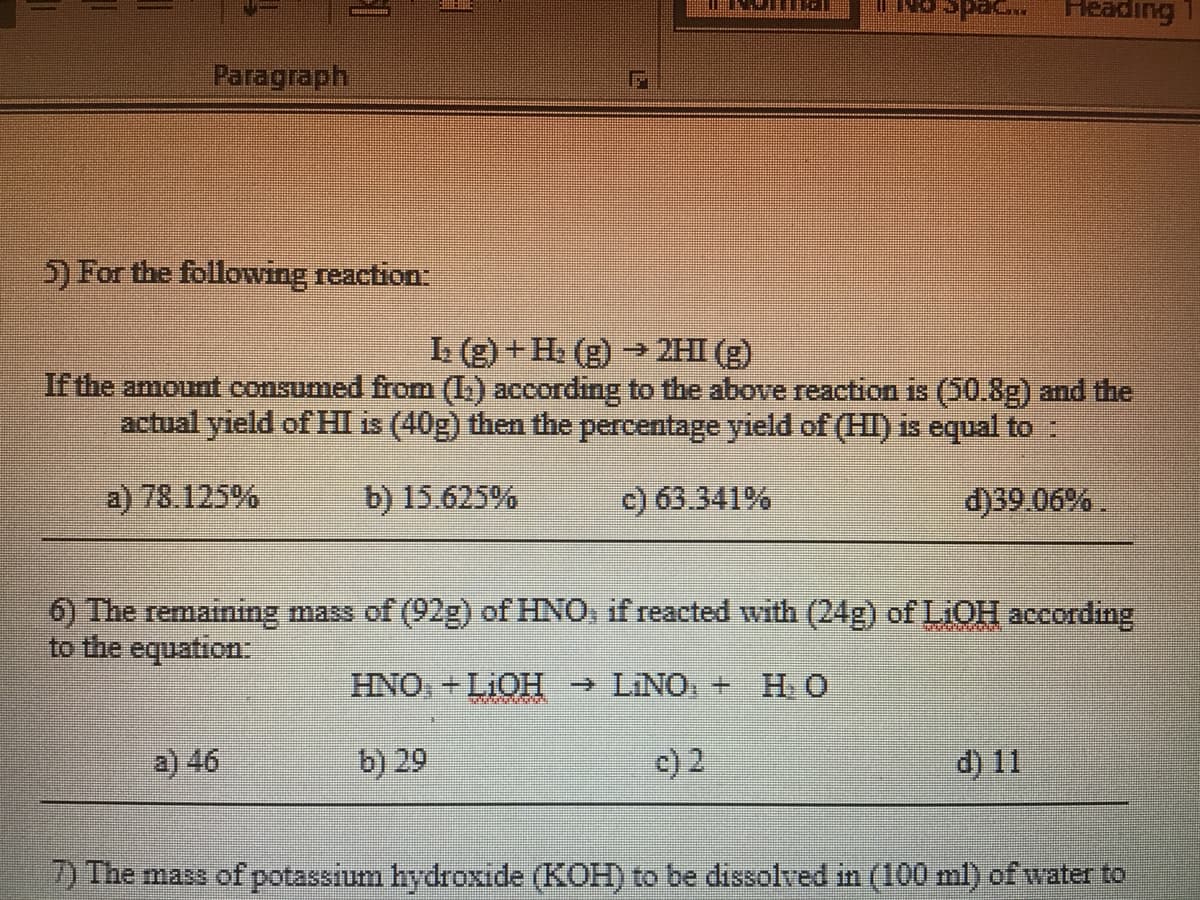
5) For the following reaction:
L (g) +H. (g) → 2HI (g)
If the amount consumed from (L.) according to the above reaction is (50.8g) and the
actual yield of HI is (40g) then the percentage yield of (HI) is equal to :
a) 78.125%
b) 15.625%
c) 63.341%
d)39.06% .
6) The remaining mass of (92g) of HNO, if reacted with (24g) of LiOH according
to the equation:
HNO+LIOH → LINO, + H. O
a) 46
b) 29
c) 2
d) 11
2) The mass of potassium hydroxide (KOH) to be dissolved in (100 ml) of water to
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning