calculate the pH at different stages of the titration using the appropriate chemical equation & formula and then select the appropriate answer. Example of answer: Between pH 8 and 9. (no decimals) A.) - Between what is the pH of the solution before any strong acid has been added? - Between what is the pH of the solution after 10.00 mL strong acid has been added? - Between what is the pH of the solution after 25.00 mL strong acid has been added?
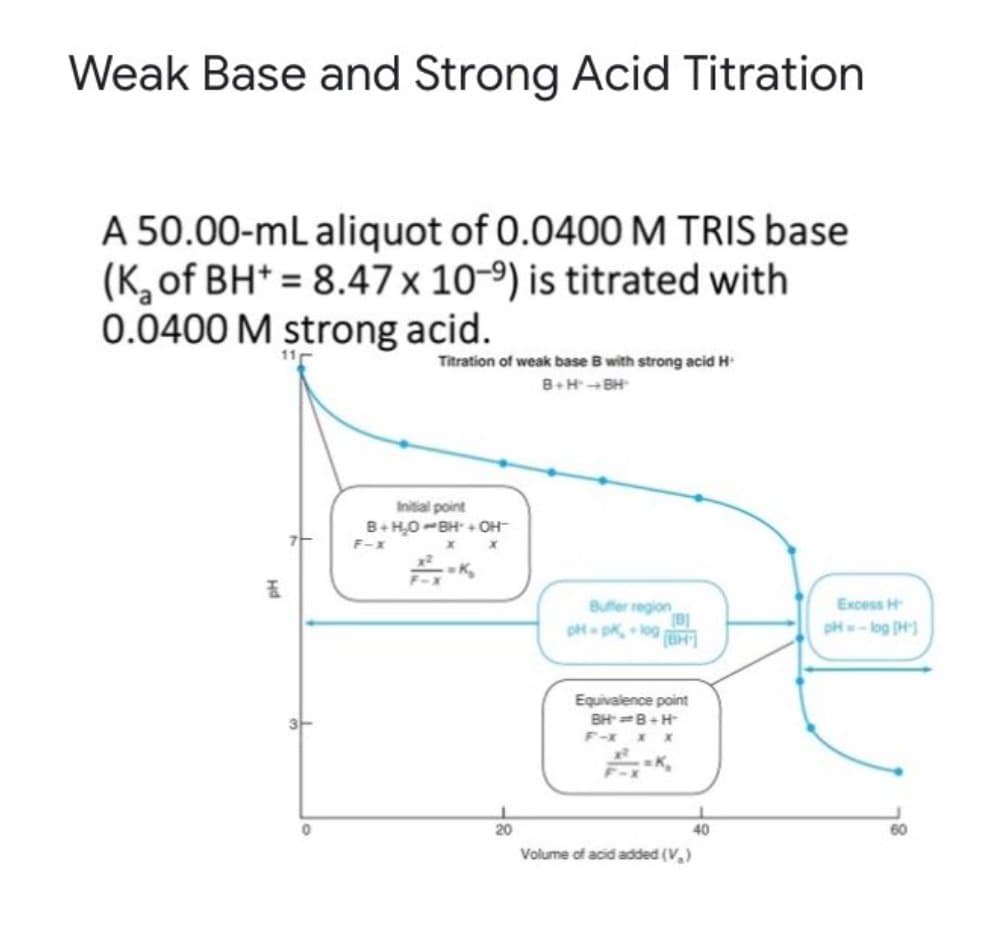
The potentiometric titration of a weak base against a stong acid is shown below. In the following problems, calculate the pH at different stages of the titration using the appropriate chemical equation & formula and then select the appropriate answer. Example of answer: Between pH 8 and 9. (no decimals)
A.) - Between what is the pH of the solution before any strong acid has been added?
- Between what is the pH of the solution after 10.00 mL strong acid has been added?
- Between what is the pH of the solution after 25.00 mL strong acid has been added?
- Between what is the pH of the solution after 40.00 mL strong acid has been added?
- Between what is the pH of the solution after 50.00 mL strong acid has been added?
- Between what is the pH of the solution after 60.00 mL strong acid has been added?
Trending now
This is a popular solution!
Step by step
Solved in 4 steps






