World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter16: Acids And Bases
Section: Chapter Questions
Problem 8STP
Related questions
Question
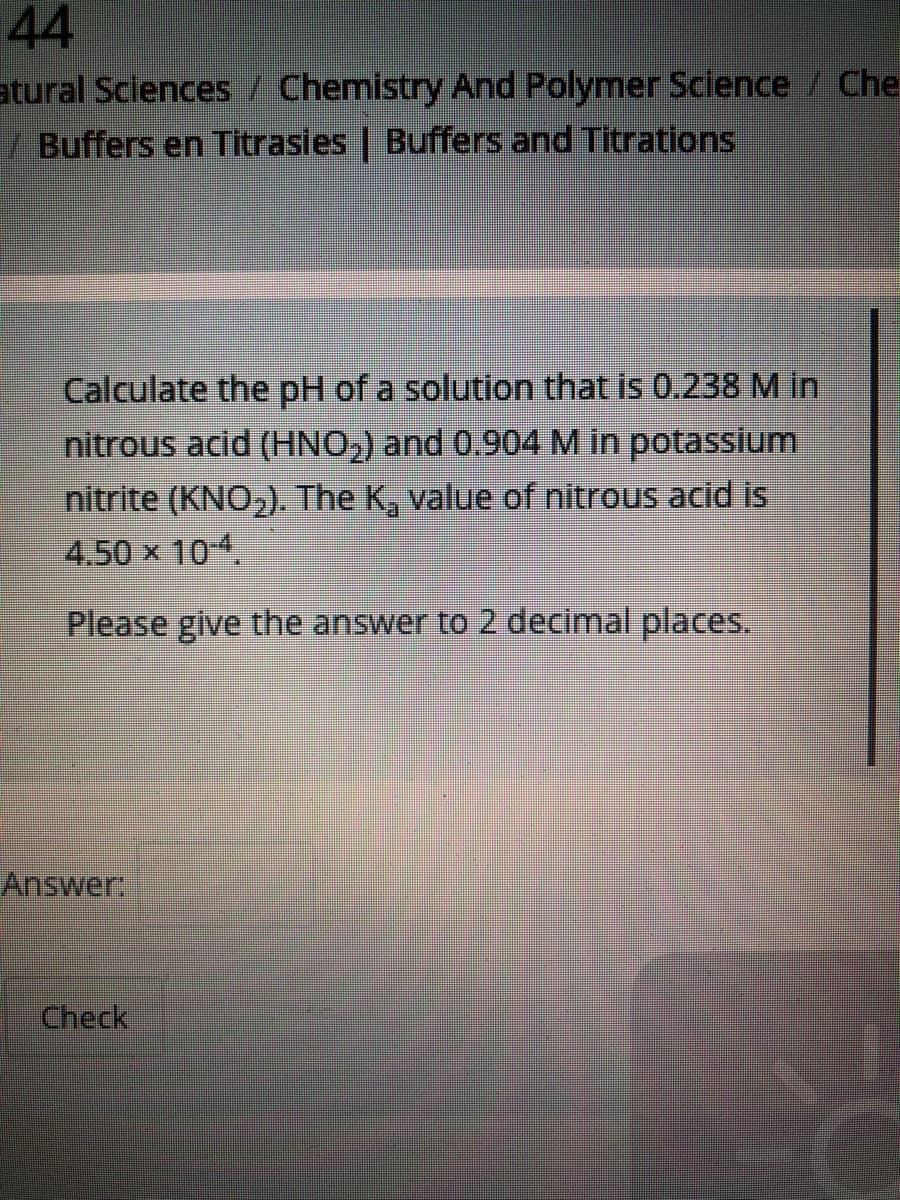
Transcribed Image Text:44
atural Sciences / Chemistry And Polymer Science / Che
/ Buffers en Titrasies | Buffers and Titrations
Calculate the pH of a solution that is 0.238 M in
nitrous acid (HNO,) and 0.904M in potassium
nitrite (KNO,). The K, value of nitrous acid is
4.50 x 104.
Please give the answer to 2 decimal places.
Answer,
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning



World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning