Calculate the pH of each of the following solutions. Ka(НС2 Н3О2) %3D 1.8 х 10 5 Ка(НCN) — 6.2 х 10 -10 Къ (NH3) — 1.8х 10-5 а. О.2 М КС2Н;О2 pH = b. 0.51 M NaCN pH = с. 0.4 М NH4CI pH =
Calculate the pH of each of the following solutions. Ka(НС2 Н3О2) %3D 1.8 х 10 5 Ка(НCN) — 6.2 х 10 -10 Къ (NH3) — 1.8х 10-5 а. О.2 М КС2Н;О2 pH = b. 0.51 M NaCN pH = с. 0.4 М NH4CI pH =
Chapter15: Complex Acid/base Systems
Section: Chapter Questions
Problem 15.15QAP
Related questions
Question
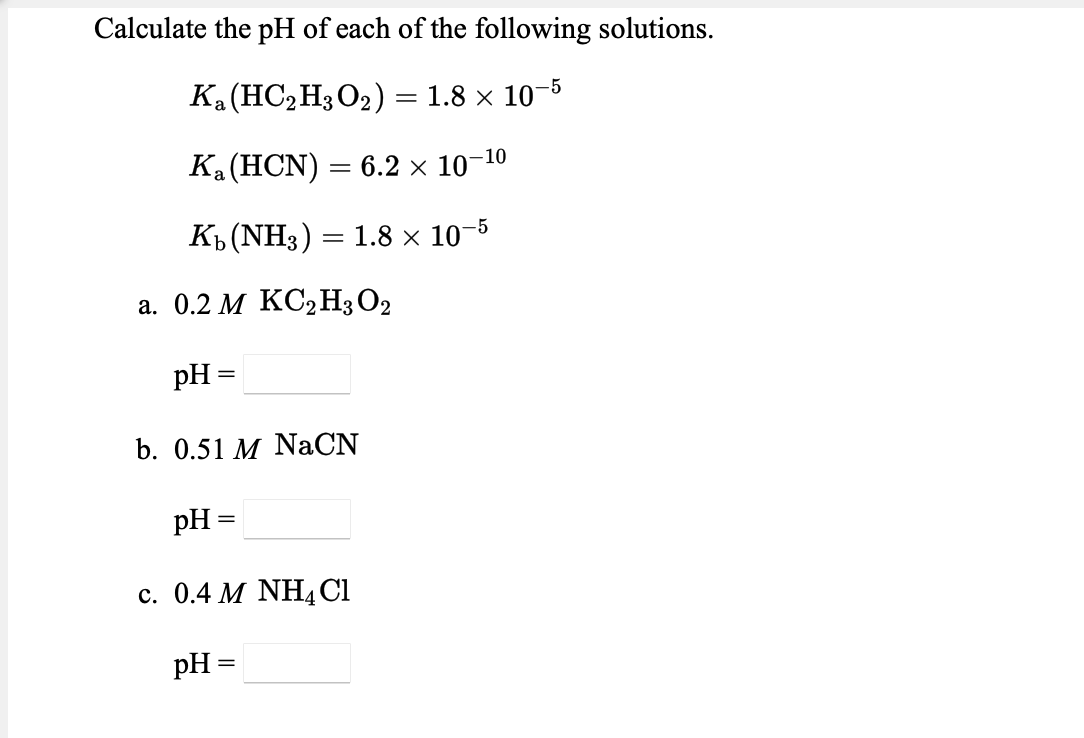
Transcribed Image Text:Calculate the pH of each of the following solutions.
Ка(НСН3О2) %3D 1.8 х 10-5
Ка (НCN)
— 6.2 х 10-10
Къ (NH3) — 1.8x 10-5
а. 0.2 М КС2Н3О2
pH
b. 0.51 M NaCN
pH =
с. 0.4 М NHACI
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning