Organic And Biological Chemistry
7th Edition
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:STOKER, H. Stephen (howard Stephen)
Chapter9: Proteins
Section: Chapter Questions
Problem 9.70EP: For the tripeptide SerArgIle which amino acid residues a. are hydrophilic b. are hydrophobic c....
Related questions
Question
Calculate the pH of the following:
0.20 M arginine hydrochloride
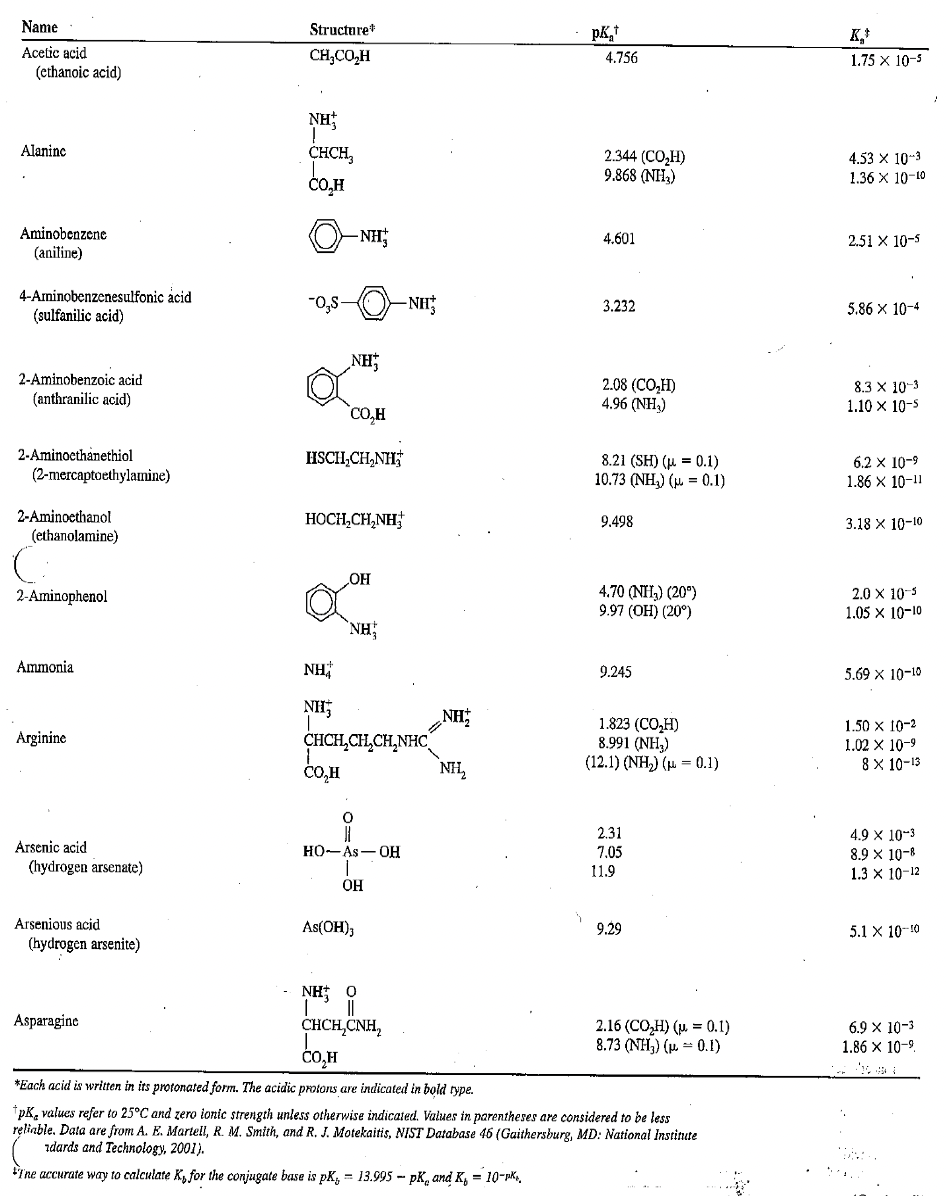
Transcribed Image Text:Name
Structure
K,
Acetic acid
CH,CO,H
4.756
1.75 x 10-5
(ethanoic acid)
NH
Alaninc
CHCH,
2.344 (CO,H)
9.868 (NH,)
4.53 X 10-3
CO,H
1.36 X 10-10
Aminobenzene
- NH
4.601
2.51 X 10-5
(aniline)
4-Aminobenzenesulfonic acid
(sulfanilic acid)
- NH
3.232
5.86 x 10-4
NH
2-Aminobenzoic acid
2.08 (CO,H)
4.96 (NH,)
8.3 X 10-3
1.10 x 10-s
(anthranilic acid)
CO,H
2-Aminocthanethiol
(2-mercaptoethylamine)
HSCH,CH,NH;
8.21 (SH) (u = 0.1)
10.73 (NH,) (p = 0.1)
6.2 x 10-9
1.86 X 10-11
2-Aminocthanol
HOCH,CH,NH
9.498
3.18 X 10-10
(ethanolamine)
4.70 (NH3) (20°)
9.97 (OH) (20°)
2.0 X 10-3
1.05 x 10-10
2-Aminophenol
`NH
Ammonia
NH
9.245
5.69 х 10-10
NH
NH
CHCH,CH,CH,NHC
1.823 (CO,H)
8.991 (NH,)
(12.1) (NH,) ( = 0.1)
1.50 x 10-2
Arginine
1.02 X 10-9
8 x 10-13
NH,
2.31
4.9 x 10-3
8.9 x 10-8
1.3 x 10-12
Arsenic acid
но-As — ОН
7.05
(hydrogen arsenate)
11.9
OH
Arsenious acid
As(OH);
9.29
5.1 X 10-10
(hydrogen arsenite)
NH: 0
||
CHCH,CNH,
co,H
Asparagine
2.16 (CO,H) (р. %3D0.1)
8.73 (NH;) (µ. - 0.1)
6.9 X 10-3
1.86 x 10-9.
*Each acid is written in its protonated form. The acidic protons are indicated in bold type.
tpK, values refer to 25°C and zero ionic strength unless otherwise indicated. Values in parentheses are considered to be less
reliable. Data are from A. E. MartelI, R. M. Smith, and R. J. Motekaitis, NIST Database 46 (Gaithersburg, MD: National Instinute
zdards and Technology, 2001).
FT'ne accurate way to calculate K, for the conjugate base is pK, = 13.995 - pK, and K, = 10-pks,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
