Calculate the physiological AG (not AG.') for the reaction: Phosphocreatine + ADP creatine + ATP Given; Phosphocreatine + H:0 creatine + Pi ADP + Pi → ATP + H;0 AG. - -43 kJ/mol AG.'-+30.5 kJ/mol at 25°C as it occurs in the cytosol of neurons, in which phosphocreatine is present at 4.7 mM, creatine at 1.0 mM, ADP at 0.20 mM, and ATP at 2.6 mM. (R = 8.315 JK" mol")
Calculate the physiological AG (not AG.') for the reaction: Phosphocreatine + ADP creatine + ATP Given; Phosphocreatine + H:0 creatine + Pi ADP + Pi → ATP + H;0 AG. - -43 kJ/mol AG.'-+30.5 kJ/mol at 25°C as it occurs in the cytosol of neurons, in which phosphocreatine is present at 4.7 mM, creatine at 1.0 mM, ADP at 0.20 mM, and ATP at 2.6 mM. (R = 8.315 JK" mol")
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter23: Organic Polymers, Natural And Synthetic
Section: Chapter Questions
Problem 46QAP: Glycolysis is the process by which glucose is metabolized to lactic acid according to the equation...
Related questions
Question
answer i only
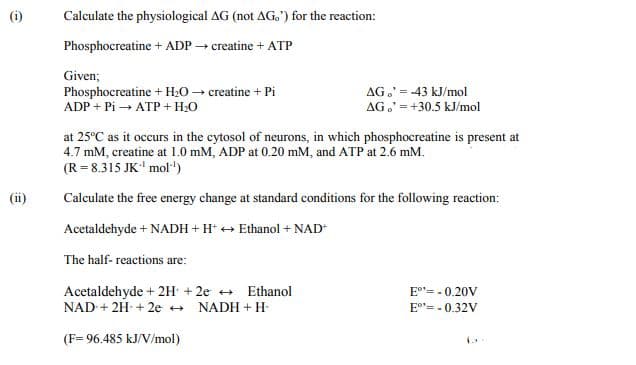
Transcribed Image Text:(i)
Calculate the physiological AG (not AG.') for the reaction:
Phosphocreatine + ADP creatine + ATP
Given;
Phosphocreatine + H20 → creatine + Pi
ADP + Pi → ATP + H:0
AG,' = -43 kJ/mol
AG.' =+30.5 kJ/mol
at 25°C as it occurs in the cytosol of neurons, in which phosphocreatine is present at
4.7 mM, creatine at 1.0 mM, ADP at 0.20 mM, and ATP at 2.6 mM.
(R= 8.315 JK' mol"')
(ii)
Calculate the free energy change at standard conditions for the following reaction:
Acetaldehyde + NADH + H* + Ethanol + NAD
The half- reactions are:
Acetaldehyde + 2H + 2e + Ethanol
NAD + 2H + 2e +
E"= - 0.20V
NADH + H-
E°"= - 0.32V
(F= 96.485 kJ/V/mol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning