Enter your answer in the provided box. 0 Calculate AG for the following reaction at 25°C. You will have to look up the thermodynamic data. 2 C2H6g)+7 02(g)4 CO2(g)+6 H2O() kJ So (JIK mol) AG'f (kJ/mol) AHf (kJ/mol) Formula 229.5 -32.89 -84.7 C2H6 219.5 68.1 52.3 CНа CO(G) 197.9 -137.3 -110.5 CO2(9) 213.6 -394.4 -393.5 CO2(aq) 121.3 -386.2 -412.9 CO32- (aq) -53.1 -528.1 -676.3 HCO3 (aq) -587.1 94.98 -691.1 HаСОз(аg) 187.4 -699.7 -623.2 69.9 HаО() -285.8 -237.2 H2O(g) 188.7 -241.8 -228.6 197.9 COG) -137.3 -110.5 213.6 CO2(g) -394.4 -393.5 121.3 CO2(aq) O2(g) -386.2 -412.9 205.0 0 0 O3(aq) 16.3 -12.09 110.88 O3(g) 163.4 142.2 237.6 e ere to search F1 F2 F3 F4 F5 F6 F7 F8 F9 #
Enter your answer in the provided box. 0 Calculate AG for the following reaction at 25°C. You will have to look up the thermodynamic data. 2 C2H6g)+7 02(g)4 CO2(g)+6 H2O() kJ So (JIK mol) AG'f (kJ/mol) AHf (kJ/mol) Formula 229.5 -32.89 -84.7 C2H6 219.5 68.1 52.3 CНа CO(G) 197.9 -137.3 -110.5 CO2(9) 213.6 -394.4 -393.5 CO2(aq) 121.3 -386.2 -412.9 CO32- (aq) -53.1 -528.1 -676.3 HCO3 (aq) -587.1 94.98 -691.1 HаСОз(аg) 187.4 -699.7 -623.2 69.9 HаО() -285.8 -237.2 H2O(g) 188.7 -241.8 -228.6 197.9 COG) -137.3 -110.5 213.6 CO2(g) -394.4 -393.5 121.3 CO2(aq) O2(g) -386.2 -412.9 205.0 0 0 O3(aq) 16.3 -12.09 110.88 O3(g) 163.4 142.2 237.6 e ere to search F1 F2 F3 F4 F5 F6 F7 F8 F9 #
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.61PAE: Estimate the temperature range over which each of the following reactions is spontaneous. (a)...
Related questions
Question
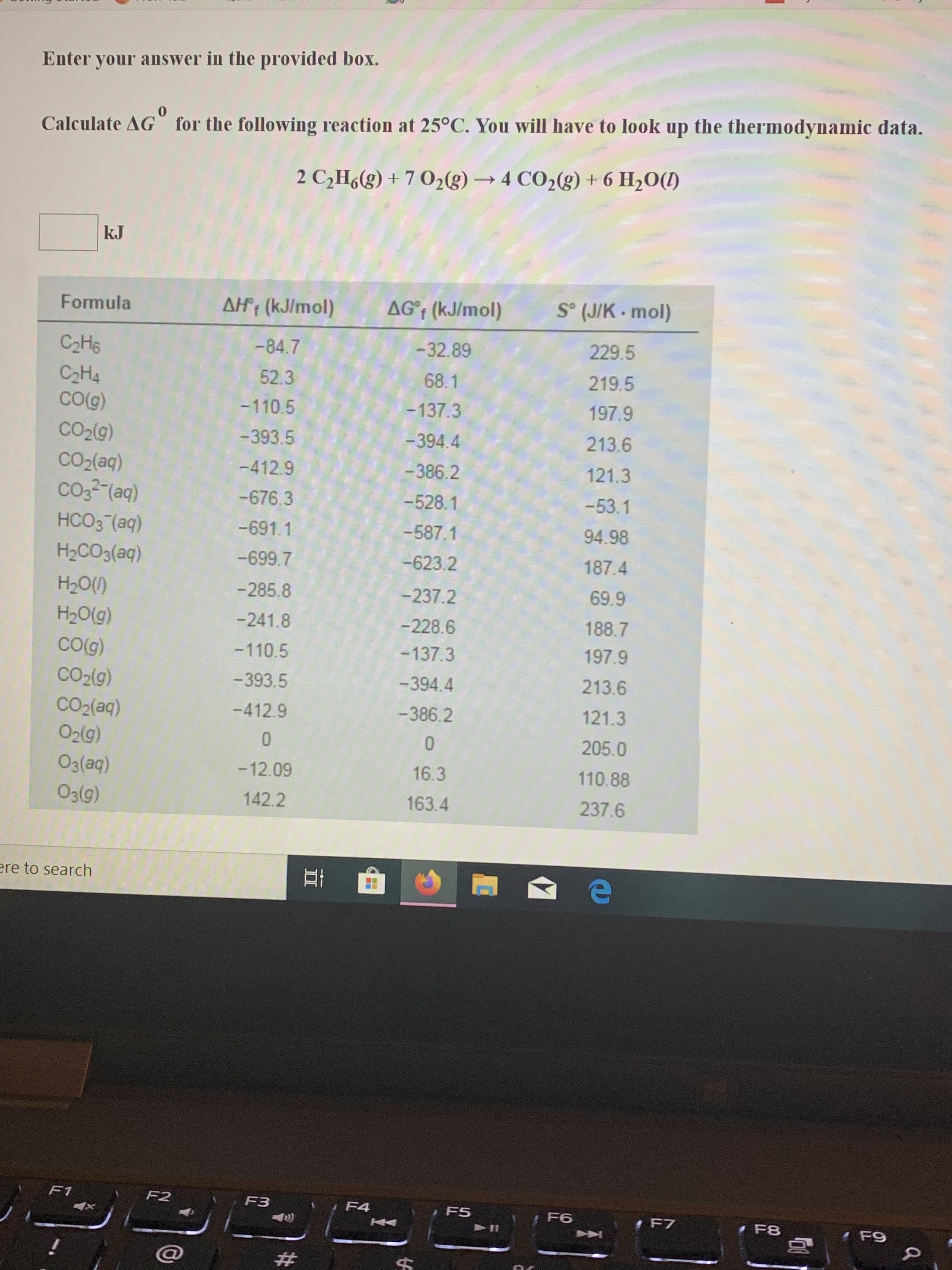
Transcribed Image Text:Enter your answer in the provided box.
0
Calculate AG for the following reaction at 25°C. You will have to look up the thermodynamic data.
2 C2H6g)+7 02(g)4 CO2(g)+6 H2O()
kJ
So (JIK mol)
AG'f (kJ/mol)
AHf (kJ/mol)
Formula
229.5
-32.89
-84.7
C2H6
219.5
68.1
52.3
CНа
CO(G)
197.9
-137.3
-110.5
CO2(9)
213.6
-394.4
-393.5
CO2(aq)
121.3
-386.2
-412.9
CO32- (aq)
-53.1
-528.1
-676.3
HCO3 (aq)
-587.1
94.98
-691.1
HаСОз(аg)
187.4
-699.7
-623.2
69.9
HаО()
-285.8
-237.2
H2O(g)
188.7
-241.8
-228.6
197.9
COG)
-137.3
-110.5
213.6
CO2(g)
-394.4
-393.5
121.3
CO2(aq)
O2(g)
-386.2
-412.9
205.0
0
0
O3(aq)
16.3
-12.09
110.88
O3(g)
163.4
142.2
237.6
e
ere to search
F1
F2
F3
F4
F5
F6
F7
F8
F9
#
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning