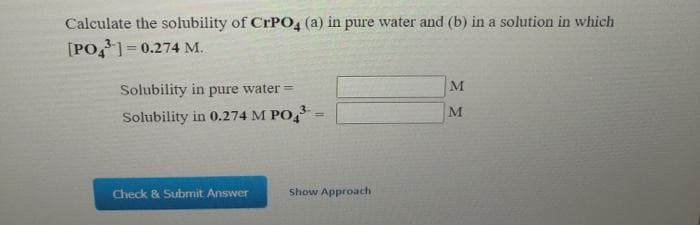
Calculate the solubility of CrPO4 (a) in pure water and (b) in a solution in which [PO,=0.274 M. %3D M Solubility in pure water = Solubility in 0.274 M PO,
Q: Calculate the solubility of copper(I) sulfide (Cu2S, Ksp = 2.26 × 10−48) in pure water.
A: The equation of dissociation of Cu2S is given below. Cu2S (s) ⇌ 2 Cu+ (aq) + S2- (aq)
Q: Pbl, solid (Kps = 9.8 x 10-9) is placed in a beaker with water. After some time, the concentration…
A:
Q: Hydroxyapatite, Ca1,(PO4),(OH)2 , has a solubility constant of Kp = 2.34 x 10-5», and dissociates…
A:
Q: Calculate the solubility of TlBrO3(s) in water in grams per liter at 25C given that Ksp=1.1x10^-12…
A: Given-> Ksp of TlBrO3 = 1.1 × 10-12
Q: How would I find the formation constant using the given information?
A: The equilibrium equation for the formation of AC5 complex and the formation constant expression is…
Q: An acidic solution of copper (.35 M Cu+2) was treated with KOH until Cu(OH)2 precipitated. At what…
A: Given : Concentration of Cu2+ = 0.35 M Using appendix, the solubility product of Cu(OH)2 is 1.6 X…
Q: Calculate the solubility of Co(OH)3 (s), Ksp = 2.5 x 10-43, in a solution at pH= 4.0
A: The equilibrium is,
Q: Calculate the solubility (in g/L) of Aluminum sulfide (Al2S3) In 0.25 M Al+3 solution. (standard…
A:
Q: The determination of cyanide ions (CN-) is carried out according to the Mohr method: a titrating…
A: The equilibrium between AgCN in solid phase and Ag+ in solutionphase is given as:AgCN (s) ⇌ Ag+ (aq)…
Q: Identify the type of coprecipitation that occurred in the following situation. (a) The…
A: answer - (a) The precipitation of bulky precipitates (e.g. MgNH4PO4) traps significant amount of the…
Q: A: Calculate the solubility product constant for PbClz, if 80.0 mL of a saturated solution of P6CI…
A: Let s be the molar solubility of PbCl2 For PbCl2, Ksp is given by: PbCl2 ↔ Pb2+ + 2Cl-…
Q: A solution was prepared by dissolving 511 mg of Na,Fe(CN), (M = 303.91 g/mol) in sufficient water to…
A: c Weight/volume percentage is- W/V%=mass of solutegvolume of solution mL×100…
Q: What is the minimum pH at which Cd(OH)2(s) will precipitate from a solution that is 0.0055M in…
A: Solubility product In case of a sparingly soluble salt, the solubility of salts are very less.…
Q: The solubility of calcium arsenate (Ca3(AsO4)2, molar mass = 398.078 g) in water is measured to be…
A: Given that: solubility of Ca3(AsO4) = 0.020 g/L Molar mass of Ca3(AsO4) = 398.078 g/mol
Q: A: Calculate the solubility product constant for P6C12, if 80.0 mL of a saturated solution of PbCl…
A: (a) A saturated solution is a solution that contains exact amount of solute the given volume of…
Q: 2. A sample of pure sodium chloride (MW = EW = 58.44 ) weighing 0.2286 g is dissolved in water and…
A: Given data : Mass of NaCl = 0.2286 g Volume of AgNO3 = 50.00 mL =…
Q: The generic metal A forms an insoluble salt AB(s) and a complex AC5(aq). = 0.100 M, [C] = 0.0340 M,…
A: a) Given: The equilibrium concentrations of species are, => [A] = 0.100 M [C] =…
Q: Calculate the molar solubility of Ca(OH)2 (Ksp = 5.02 x 10) in %3D solution that is buffered at pH=…
A:
Q: Calculate the molar solubility of CaF, at 25°C in a 0.010 M NaF solution. (Ksp CaF2 = 3.9 x 10 11)…
A:
Q: 1. In a redox titration, 12.50 mL of 0.800 mol/L K2Cr2O7 (aq) was used in an acidic solution to…
A: Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: Consider the insoluble compound copper(II) carbonate , CUCO3 . The copper(II) ion also forms a…
A:
Q: platinum in this solution as well as b) the molar concentration of the platinum in this solution.…
A: volume by volume percentage of the solution is the way to quantify the amount of the solute present…
Q: Calculate the solubility, in mol/L, when lead (1) bromide (Ksp = 4.0 x 105) dissolves in an aqueous…
A:
Q: Compare the solubility of nickel(II) sulfide in each of the following aqueous solutions: Clear All…
A:
Q: 67. What is the pH at which 0.01 M Co2 ions in solution just precipitate as Co(OH), ? Kp of Co(OH),…
A: We have to find the pH at which Co+2 ion in solution precipitates
Q: Calculate the solubility product constant for PbCl2,
A: Solubility product is the product of concentrations of products raised to the power of their…
Q: Calculate the solubility of copper(I) sulfide (Cu2S, Ksp = 2.26 × 10−48) in a 0.010 M CuC2H3O2(aq)…
A: The solubility of copper (I) sulfide in 0.01 M copper (I) acetate solution can be expressed as…
Q: Calculate the solubility of Zn(OH)2(s) in 2.0 M NAOH solution. (Hint: You must take into account the…
A: Solubility product: The solubility of a sparingly soluble salt-forming saturated solution in water…
Q: The solubility of calcium arsenate (Ca3(AsO4)2, molar mass = 398.078 g) in water is measured to be…
A: given solubility in g/L. If we want convet solubility into molar solubility Simply divide the…
Q: Calculate the molar solubility of CaF2 at 25oC in 0.010M Ca(NO3)2 solution. (Ksp = 3.1 x 10-5 M)
A: The molar solubility of CaF2 in 0.010M Ca(NO3)2 solution has to be determined. Given: The Solubility…
Q: Ni content of the steel can be determined by the precipitation gravimetric analysis. For this…
A: The complex of Ni forming is Ni(DMG)2
Q: The generic metal A forms an insoluble salt AB(s) and a complex AC5(aq). The equilibrium…
A:
Q: The generic metal A forms an insoluble salt AB(s) and a complex AC5(aq). The equilibrium…
A: he generic metal A forms an insoluble salt AB(s) and a complex AC5 (aq). The equilibrium…
Q: Calculate the solubility (in g/L) of Aluminum
A:
Q: Calculate the solubility of Co(OH)3, Ksp=2.5*10^-43, in a solution at PH=4.0
A: The solubility reaction is given by Co(OH)3 (s) ------> Co3+ (aq) + 3 OH- (aq) since pH = 4…
Q: The equilibrium concentrations in a solution of AC5 were found to be [A] = 0.100 M, [C] = 0.0240 M,…
A: Complex formation reaction: A+5C↔AC5 …Kf Solubility equilibrium reaction: AB(s)↔A+B …Ksp
Q: The solubility of calcium arsenate (Ca3(AsO4)2, molar mass = 398.078 g) in water is measured to be…
A: The solubility (S) of calcium arsenate [(Ca3(AsO4)2 is 0.012 g L-¹. The molar mass of calcium…
Q: Chromium(III) hydroxide, Cr(OH)3, is highly water insoluble. Its molar mass is 103.02 g/mol. a.)…
A: As you have posted multiple parts and have not mentioned which part you want to be solved. Hence we…
Q: Calculate the ionic strength (u) of a solution containing silver chromate Ag2CrO4 in 0.100 M sodium…
A: Given: Molarity of NaNO3 = 0.1 M Ksp of Ag2CrO4 = 1.2 x 10-12 Ionic strength formula is: where u…
Q: The gravimetric factor (GF ) for Ag in precipitated Ag2CrO4 equal:(Atomic (weights, Cr=24;Ag=108…
A: The reaction for the process is : 2Ag+ + CrO42- ⇌ Ag2CrO4(ppt.) The Gravimetric factor, (GF) can…
Q: Formula of a compound 261.0 mg of FeCl,-xH,O crystals were dissolved in a diluted hydrochloric acid…
A: FeCl2.xH2O or Fe2+(aq) is oxidized by KMnO4(aq) to FeCl3 or Fe3+(aq). KMnO4(aq) or MnO4-(aq) itself…
Q: Consider the equilibrium reaction: YZ2(s) ⇌ Y2+(aq) + 2Z-(aq). What is the molar solubility of YZ₂…
A: The molar solubility, which is directly related to the solubility product, is the amount of moles…
Q: (a) If the molar solubility of Cu3(PO4)2 at 25 oC is 1.67e-08 mol/L, what is the Ksp at this…
A:
Q: Calculate the mass solubility of Ag2CO3 (Ksp= 8.1 x 10^-12, MM = 275.74 g/mol) in a 0.010 M AgNO3…
A: Given: Ksp of Ag2CO3= 8.1 x 10^-12, MM of Ag2CO3 = 275.74 g/mol Concentration of AgNO3…
Q: Which of the following is soluble in water at 25∞C a) FeCO3 c) Fe(OH)2 b) Fe3(PO4)2 d)…
A: Given Four ferrous salts such as FeCO3, Fe(OH)2, Fe3(PO4)2 and Fe(NO3)2, from whom the most soluble…
Q: What is the solubility, s, of CaF2 (Ksp = 3.4 x 10 11) in a 0.35 M CaCl, solution? %3D
A:
Q: Determine the solubility of PBF2, which has Ksp= 4.0 x 108.
A:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- Sulfide ion (S2- ) is formed in wastewater by the action of an aerobic bacteria on organic matter. Sulfide can be readily protonated to form volatile, toxic H2S. In addition to the toxicity and noxious odor, sulfide and H2S cause corrosion problems because they can be easily converted to sulfuric acid when conditions change to aerobic. One common method to determine sulfide is by coulometric titration with generated silver ion.At the generator electrode, the reaction is Ag Ag+ + e-. The titration reaction is S2- + 2Ag+ Ag2S(s). (a) A digital chloridometer was used to determine the mass of sulfide in a wastewater sample. The chloridometer reads out directly in ng Cl-.In chloride determinations, the same generator reaction is used,but the titration reaction is Cl- + Ag+ AgCI(s). Derive an equation that relates the desired quantity, mass S2- (ng), to the chloridometer readout in mass Cl- (ng). (b) A particular wastewater standard gave a reading of 1689.6 ng Cl-. What total charge in coulombs was required to generate the Ag+ needed to precipitate the sulfide in this standard? (c) The following results were obtained on 20.00-mL samples containing known amounts of sulfide.17 Each standard was analyzed in triplicate and the mass of chloride recorded. Convert each of the chloride results to mass S2- (ng). (d) Determine the average mass of S2- (ng), the standard deviation, and the %RSD) of each standard. (e) Prepare a plot ofthe average mass of S2- determined (ng) versus the actual mass (ng). Determine theslope, the intercept, the standard error, and the R2 value. Comment on the fit of the data to a linear model. (f) Determine the detection limit (ng) and in parts per million using a k factor of 2 (see Equation 1-12). (g) An unknown wastewater sample gave an average reading of 893.2 ng Cl. What is the mass of sulfide (ng)? If 20.00 mL of the wastewater sample was introduced into the titration vessel, what is the concentration of S2- n parts per million?What is the concentration of ions in the soil solution after fertilizer application? Suppose that 122 pounds of K+ were applied per acre, then a gentle rain soaked the top 10 inches of soil to field capacity, which for the given soil was about 16% water by volume. If the K+ was applied as KCl, it is plausible that it all dissolved and distributed relatively uniformly with the infiltrating water. If so, then what was the K+ concentration in the soil solution in mol K+/L solution? Note that the volume can be computed like we do for an acre-furrow-slice (AFS), as area times depth. This is going to be a relatively small number, so please report your answer in mol K+/L solution to at least 5 decimal places.Solubility question again! Calculate the solubility (in g/L) of Aluminum sulfide (Al2S3) in .1 M H2S solution (standrard conditions) {Ksp for Al2S3 is 1.5×10-27}.
- Experiment4: Solubility Product In the experiment to determine the solubility of KI04, in water, the following observations are made at a certain temperature: Volume of saturated KI04 solution taken in the conical flask = 25.0 mL KI added=2g H2SO4 (3.0 M ) added = 20.0 mL. Volume of 0.200M sodium thiosulphate used for titration = 8.00 mL Q1.If the mean ionic activity coefficient, γ± of KIO4 in the saturated solution is 0.85, what is Ksp of KIO4.?Please explain.. Pls do fast ..its urgent.. i will give like for sure Solution must be in typed formAngeline performed an experiment on vitamin C content. She purchased bell peppers of different colours from local wet market. Bell pepper samples were cut, blended and homogenized with a fixed portion of water. Subsequently, coulometric titration was performed using iodine under constant current of 60 mA. In one of the experiment replicate,starch end point was achieved after 18 minutes. Suppose that bell pepper sample recorded initial mass of 115 g, deduce it’s vitamin C (176 g/mol) content in percentage. (F = 96500C)
- A 50.00 mL volume of 0.0600M K2CrO4 is mixed with 50.00 mL 0.0800 M AgNO3. Calculate the concentration of Ag+, CrO42-, K+, and NO3- at equilibrium. The solubility product of Ag2CrO4(s) is 1.20 x 10-12. Please show workDetermine the solubility of KIO4 in water. Volume of saturated KIO4 in conical flask is 25.0 ml,KI is 2g,H2SO4 (3.0M) is 20.0 ml added to KI solution , volume of 0.200 M sodium thiosulphate used for titration is 8.00 ml.and if mean ionic activity co efficient,y+- of KIO4 in the saturated solution is 0.85 ,what is Ksp. .Solid cobalt (II) acetate is slowly added to 125 mL of 0.0945 M ammonium chromate solution. What is the concentration of cobalt required to just initiate precipitation? The Ksp of CoCrO4 is 7.1 * 10 -4. Report answer in scientific notation to two sig figs.
- PS. Further values required for the solvings are give in the various situations below. (ANSWER) Situation: A community in a mountainous area of Bohol uses water collected from a nearby natural spring. A sample was submitted to a laboratory for the analysis of its total hardness. Required: SHOW YOUR COMPLETE CALCULATIONS. BASED ON THE IMAGE PROVIDED BELOW FOR THIS QUESTION: Calculate the amount of titrant used in each trial to reach endpoint. Report total hardness of the sample as mean ±sd. a. 250.0 mL of 500.0 ppm of CaCO3 solution from a primary standard (assume solvent is distilled water only). Answer : Mass of CaCO3 = 0.125 g b, The EDTA solution was standardized by titrating it with a 25.0 mL aliquot of the CaCO3 solution. How much of the titrant was consumed. Answer: Volume of EDTA consumed = 12.405 g c. Calculate the average titer (mg CaCO3/mL EDTA). Mass of CaCO3 in 25 mL CaCO3 solution: 0.0125 g Answer: 1.008 mg CaCO3/ mL EDTADetermine the Ksp of PbBr2 if its molar solubility in water at 25 °C is 1.05 x 10−2 M. You can use either Method 1 or 2. Note: This equation, (S = n+m√Ksp/nn . Mm), can be rearranged to the following to solve for Ksp; Ksp = S(n+m) . nn.mmThe concentration of CO in the air can be determined by passing a known volume of air through a tube containing I2O5, resulting in the formation of CO2 and I2. I2 is removed from the tube by distillation and collected in a solution containing excess KI, producing I3-. I3- is titrated with a standard solution of Na2S2O3. A 4.79 L air sample was sampled as described here, requiring 7.17 ml of 0.00329 M Na2S2O3 to reach the endpoint in a typical analysis. If the density of the air is 1.23×10^-3 g/ml, what is the amount of CO in the air in ppm? (CO: 28 g/ml)



