g The solubility of CuCO 3 in water at 25 °C is measured to be 0.0019 Use this information to calculate K sp for CuCO3. Round your answer to 2 significant digits. x10 ☑ Calculate the solubility of Zn(OH), in water at 25 °C. You'll find Ks sp Round your answer to 2 significant digits. ㅁ은 data in the ALEKS Data tab.
g The solubility of CuCO 3 in water at 25 °C is measured to be 0.0019 Use this information to calculate K sp for CuCO3. Round your answer to 2 significant digits. x10 ☑ Calculate the solubility of Zn(OH), in water at 25 °C. You'll find Ks sp Round your answer to 2 significant digits. ㅁ은 data in the ALEKS Data tab.
Chapter9: Aqueous Solutions And Chemical Equilibria
Section: Chapter Questions
Problem 9.8QAP
Question
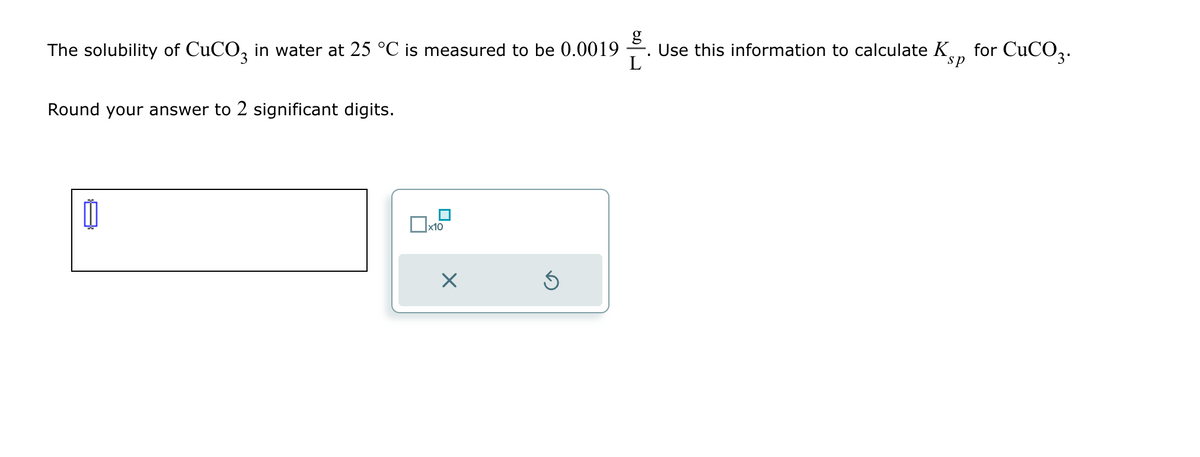
Transcribed Image Text:g
The solubility of CuCO 3 in water at 25 °C is measured to be 0.0019
Use this information to calculate K
sp
for CuCO3.
Round your answer to 2 significant digits.
x10
☑

Transcribed Image Text:Calculate the solubility of Zn(OH), in water at 25 °C. You'll find Ks
sp
Round your answer to 2 significant digits.
ㅁ은
data in the ALEKS Data tab.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning