Calculate the specific heat capacity at constant volume of water vapor, assuming the nonlinear triatomic molecule has three translational and three rotational degrees of freedom and that vibrational motion does not contribute. The molar mass of water is 18.0 g/mol. Express your answer in J/(kg · K).
Calculate the specific heat capacity at constant volume of water vapor, assuming the nonlinear triatomic molecule has three translational and three rotational degrees of freedom and that vibrational motion does not contribute. The molar mass of water is 18.0 g/mol. Express your answer in J/(kg · K).
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 12CQ: Experimentally it appears that many polyatomic molecules' vibrational degrees of freedom can...
Related questions
Question
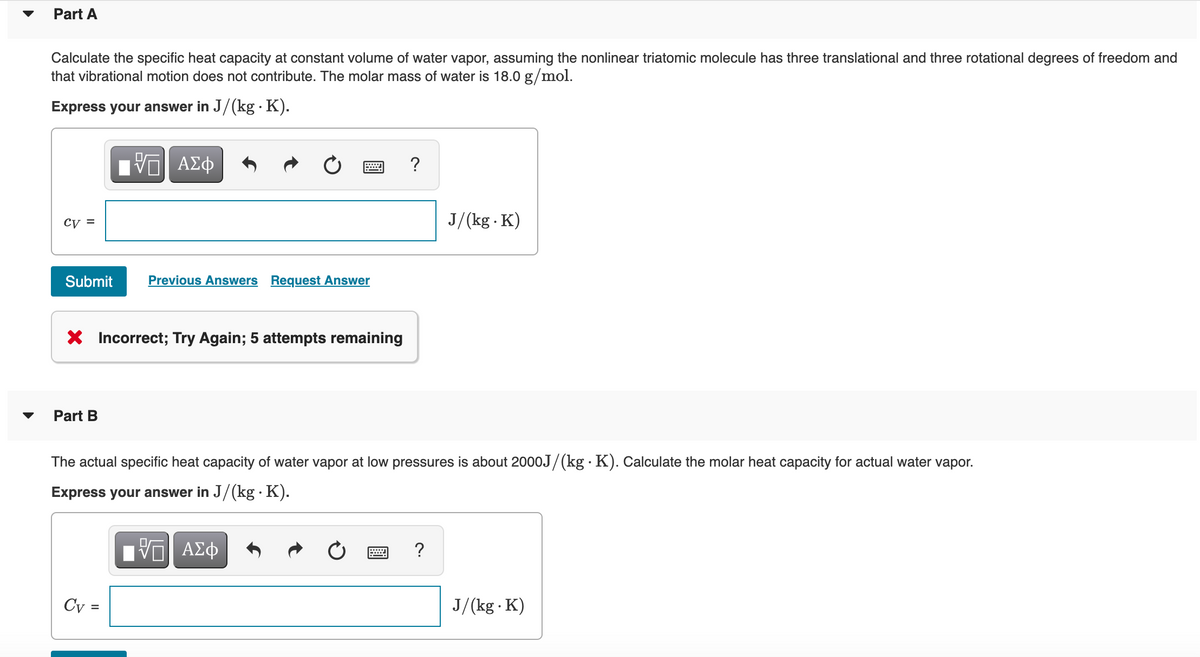
Transcribed Image Text:Part A
Calculate the specific heat capacity at constant volume of water vapor, assuming the nonlinear triatomic molecule has three translational and three rotational degrees of freedom and
that vibrational motion does not contribute. The molar mass of water is 18.0 g/mol.
Express your answer in J/(kg · K).
?
cy =
J/(kg K)
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
Part B
The actual specific heat capacity of water vapor at low pressures is about 2000J/(kg · K). Calculate the molar heat capacity for actual water vapor.
Express your answer in J/(kg · K).
ΑΣφ
?
Cy =
J/(kg - K)
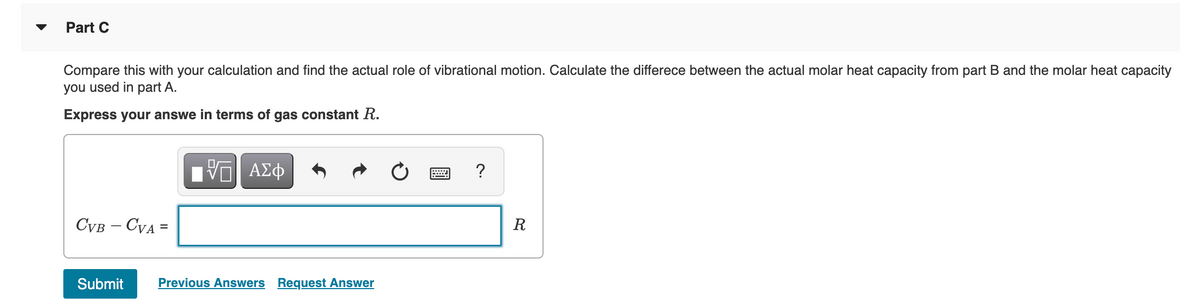
Transcribed Image Text:Part C
Compare this with your calculation and find the actual role of vibrational motion. Calculate the differece between the actual molar heat capacity from part B and the molar heat capacity
you used in part A.
Express your answe in terms of gas constant R.
?
CVB – CVA =
R
%3D
Submit
Previous Answers Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning