Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: Given that : We have to calculate the standard reaction free energy of the following reaction :…
Q: 11.8 Use Appendix E to determine whether each reaction is spontaneous under standard conditions at…
A: Hi, as you have not provided Appendix E, so we are using google for standard Gibb’s energy of…
Q: Consider the reaction: 2NO(g) + O2(g)–→2NO2(g) Using standard thermodynamic data at 298K, calculate…
A: Explanation
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: The given reaction is - - > 4PCl3 (g) - - > P4 (g) + 6Cl2 (g) They standard free energy…
Q: Consider the decomposition of yellow mercury (II) oxide. HgO (s,yellow) ⟶ Hg(l) + 1/2 O2(g)…
A: Given that, the decomposition of yellow mercury (II) oxide HgO (s, yellow) ⟶ Hg(l) + 12O2(g). Also,…
Q: Calculate the free energy change for the following reaction and predict whether the reaction occurs…
A:
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: Standard ∆G values - CH3OH = -162.0 KJ/mol CO = - 137.2 KJ/mol HCH3CO2 = -389.0 KJ/mol
Q: 18.41 Calculate the standard free energy of the following reactions at 25 degrees * C , using…
A: The details solution for this is provided below in attach image.
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: It is an example of Hess's Law Here we are required to find the standard reaction free energy of the…
Q: Consider the reaction 2H,O1) 2H,(g) + 0,(g) The standard free energy change for this reaction is…
A: The amount of energy released during the conversion of reactant into the product under the standard…
Q: Consider the reaction:2CO(g) + O2(g)2CO2(g)Using standard thermodynamic data at 298K, calculate the…
A: Gibb’s free energy of mixing (∆Grxn) of gas– The change in Gibbs free energy due to mixing of two…
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: Given reaction: 2Als + Fe2O3s → Al2O3s + 2 Fes
Q: 8 Find the correct thermodynamic description of the following reaction that is spontaneous at some…
A: Introduction: Gibbs free energy determine the maximum amount of work done in a system at constant…
Q: Consider the reaction: Co(g) + 3H2(g)–CH4(g) + H20(g) Using standard thermodynamic data at 298K,…
A:
Q: Using the values for standard Gibb's Free Energy in the table, calculate the Gibb's Free Energy for…
A: Given reaction: A + 3B = 2C + D Free energy (Gibbs free energy) is the term that is used to explain…
Q: The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning…
A: Interpretation: We have to discuss if the reaction is exothermic , in that case which condition from…
Q: Use the thermodynamic information below to determine the free energy change for the following…
A: fullscreen
Q: Consider the reaction: CO(g) + H20(1) CO2(g) + H2(g) Using standard thermodynamic data at 298K,…
A: The equation is; ∆Go = ∑nGfo(product) - ∑nGfo(reactant) Where, ∆Go = free energy change ∆Gof =…
Q: Consider the reaction: Co2(g) + H2(g)→CO(g) + H,0(g) Using standard thermodynamic data at 298K,…
A: ∆G°rxn = ∆G°f(products ) - ∆G°f(reactants )
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: Standard Free energy of reaction:- The standard free energy change (∆Gº) of a chemical…
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A:
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: Standard reaction free energy is represented as ∆G0. It is the difference between the standard free…
Q: 25. From the appropriate absolute entropies and heats of formation calculate the standard free…
A: Change in enthalpy (H) = Enthalpy of product - Enthalpy of reactant Change in entropy (S) = Entropy…
Q: predict whether the reaction would be spontaneous
A: A reaction is said to be spontaneous reactions if it favors the formation of products at the…
Q: A reaction with ∆S > 0 is spontaneous at high temperature. Under what conditions would it also be…
A: Reaction is spontaneous with ∆S > 0 at high temperature. The conditions at which reaction is…
Q: Consider the following reaction at 25 °C: 3 NiO(s) +2 NH3(g)→ 3 Ni(s) + N2(g) + 3 H2O(g) If AH° =…
A: The relationship between standard enthalpy (∆H), entropy (∆S) and free energy (∆G) at a particular…
Q: reaction has AHxn= 533 kJ/mol and ASTxn= -57 J/K•mol. What can be said about this %3D action? The…
A: Answer
Q: What is the standard free energy change, A°G, for the following reaction at 298K? C;H,OH(1) + 30:(g)…
A: Consider the given reaction is as follows; C2H5OH l + 3 O2 g ⇄ 2 CO2 g + 3 H2O g The…
Q: Calculate the free energy change in kJ for the following reaction at 25 °C. 3 A(g) + B(g) – 4 C(g)…
A: A question based on thermodynamics, which is to be accomplished.
Q: NH3 (g) +3 F2(g) → NF3(g) + 3 HF(g) Compound AG" † (kJ/mol) NH3(9) -16 F2(9) NF3(9) -91 HF(9) -280
A: To check the reaction given is thermodynamically favoured or not NH3 + 3F2 = NF3 + 3 HF
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: Given, MgCl2(s) + H2O(l) ----> MgO(s) + 2HCl(g) Species standard gibbs free energy (∆G°f)…
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: Since, Free energy of the reaction is the difference of the sum of the gibbs free energy of product…
Q: Consider the reaction 2H2S(g) +302(g) =2H20(g) + 2SO2(g). If the standard free energy of the…
A:
Q: A student determines the value of the equilibrium constant to be 3.88×1013 for the following…
A: The reaction given is 4 HCl (g) + O2 (g) ------------> 2 H2O (g) + 2 Cl2 (g)…
Q: Why is it important in using change in free energy, ΔG, as the thermodynamic parameter for assessing…
A: Thermodynamics is branch of chemistry which deals with heat gained and heat lost by system.
Q: Suppose a reaction has DH > 0 and DS > 0; that is, both enthalpy and entropy changes have positive…
A: The Gibbs-Helmholtz equation is: ∆G = ∆H - T∆S. If ∆G is negative then the reaction will be…
Q: Without doing a numerical calculation, determine which of the following will reduce the free energy…
A: The reaction with more negative value of free energy at higher temperature has to be given.
Q: https://openstax.org/books/chemistry-atoms-first-2e/pages/g-standard-thermodynamic-properties-for-se…
A: ΔG°f (KJ / mol) NF3(g) -524.53 F2(g) 0 N2(g) 0
Q: Calculate the standard free energy change for the combustion of 1 mole methane using standard free…
A: Given that :
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: The given balanced chemical reaction is, 2Als+Fe2O3s→Al2O3s+2Fes The oxidation half reaction is…
Q: Consider the reaction: 2CO(g) + 2NO(g)2CO2(g) + N2(g) Using standard thermodynamic data at 298K,…
A: Free energy change for a reaction is calculated from the change of formation energy. If ∆G is…
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A: Given reaction is : 2Al (s) + Fe2O3 (s) ------> Al2O3 (s) + 2Fe (s) Calculate the standard change…
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A:
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A:
Q: A student determines the value of the equilibrium constant to be 3.80×1013 for the following…
A: In order to derive the free energy of any specific system, apply the equation between free energy…
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A:
Q: The following reaction is endothermic. 2NH3(g) → N2(g) + 3H2(g) This means the reaction A.…
A:
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A:
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A:
Q: Which of the following does the change in the free energy of a reaction predict? A) the work done B)…
A: Free energy is a deciding factor for the spontaneity of any chemical reaction. Lower free energy of…
Calculate the standard free energy for the reaction (you may use any valid technique - there may be more than one way to do a question like this):
Al2O3(s)) + 3 H2(g) → 2 Al(s) + 3 H2O(g)
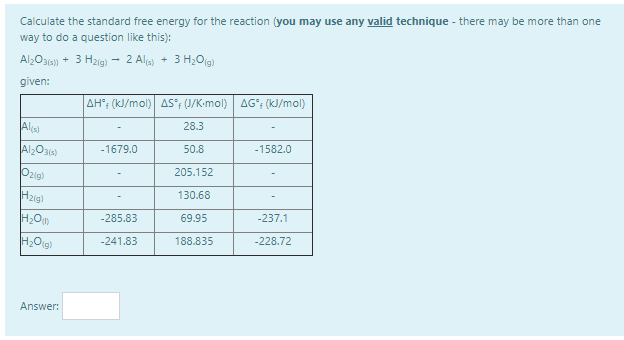
Step by step
Solved in 2 steps

- Calculate the standard free energy for the reaction at 340.0°C. (you may use any valid technique - there may be more than one way to do a question like this):Consider the reaction in the attached image: a. The standard Gibbs free energy (ΔfG˚) for this reaction (up to two decimal places) is ____________ kJ/mol. b. If the concentration of AMP is adjusted to 1.5M and that of adenosine and phosphate is adjusted to 0.50M, the actual free energy change (ΔG) (up to two decimal places) is equal to ___________ kJ/mol.The standard state free energy of hydrolysis of acetyl phosphate is ΔGo= -42.3 KJ/mol. T=20 degrees C Calculate the free energy change for the acetyl phosphate hydrolysis in a solution of 3.5 mM acetate, 3.5mM phosphate (Pi), and 4.00 nM acetyl phosphate.
- The standard free energy (DGo) for a reaction at 298 K is -104 J / mol. What is Keq for this reaction?Use the free energies of the formation given below to calculate the equilibrium constant (Keq) for the following reaction at 298 K. 2 AXZ3 (aq) + XZ (g) → 3 XZ2 (g) + A2Z (l) Keq = ? Using the following Data: AXZ3 (aq) XZ (g) XZ2 (g) A2Z (l) ΔG°f (kJ/mol) = -110.9 87.6 51.3 -237.1A student determines the value of the equilibrium constant to be 06×106for the following reaction.3Fe2O3(s) + H2(g)2Fe3O4(s) + H2O(g)Based on this value of Keq:G° for this reaction is expected to be (greater, less) _______ than zero. Calculate the free energy change for the reaction of 1.62 moles of Fe2O3(s) at standard conditions at 298K. G°rxn = _____ kJ
- A student determines the value of the equilibrium constant to be 6.61×^3 for the following reaction Co(g)+h2o(l)---------> co2(g)+ h2(g) Based on this value of Keq delta G for this reaction is expected to be (greater/ less) -------- than zero Calculate the free energy change for the reaction of 1.98 miles of co(g) at standard conditions at 298k delta G rnx = -----kjshow by calculation which direction the reaction would be energetically spontaneous, use m(Si-30)=29.973772 and m(Ni-60)=59.930789: 2(30)Si <-> (60)NiThe thermodynamic values from part A will be useful as you work through part B: ΔH∘rxn 243.5kJ/mol ΔS∘rxn 172.0J/(mol⋅K) Calculate the equilibrium constant for the following reaction at room temperature, 25 ∘C BaCO3(s)→BaO(s)+CO2(g)
- For the reaction2Na(s) + 2H2O(l)2NaOH(aq) + H2(g)H° = -368.6 kJ and S° = -15.3 J/KThe standard free energy change for the reaction of 1.55 moles of Na(s) at 259 K, 1 atm would be kJ.This reaction is (reactant, product) fill in the blank 2 favored under standard conditions at 259 K.Assume that H° and S° are independent of temperature.Use the data provided below to estimate (a) the temperature at which CaCO3 decomposes spontaneously assuming ΔrH and ΔrS remain unchanged at the temperature region of interest. CaCO3 (s) --> CaO (s) + CO2 (g) ΔfHo -1207 -635 -394 kJ mol-1 Smo 93 40 214 J K-1 mol-1 Tspont. >= ________ K. 3 s.f.Given below (c): Standard gibb's free energies (∆Gf0 kJ mol-1 ): UO2 = -962.7 UO22+ = -953.5 U4+ = -579.1 Fe2+ = -78.9 Fe(OH)3 ferrihydrite = -692.07 Mn2+ = -288.1 MnO2 pyrolusite = -465.1 HS- = 12.1 H+ = 0 H2O = -237.1 S0 = 0 Given: U(VI) as uraninite; UO2 (where Mn2+ = reductant; MnO2 pyrolusite = product): ∆ Gr0 = -21.3 KJ/mol E0 (emf) = 0.110 V n = 2 F = 96.42 QUESTION: Calculate Eh equation below to calculate at different pH: – Eh = E0 + (RT/nF) * lnK For U(VI) as uraninite; UO2 (where HS- = reductant; S0 = product): UO22+ + Hs- ---- > UO2 + S + H+ [A] pH 3 [B] pH 7

