Calculate the theoretical yield in grams All, from the complete reaction of 113 grams l according to the following balanced chemical equation: 2 Al(s) + 3 12(s) 2 All (s)
Calculate the theoretical yield in grams All, from the complete reaction of 113 grams l according to the following balanced chemical equation: 2 Al(s) + 3 12(s) 2 All (s)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.93PAE: 3.93 Adipic acid is used in the production of nylon, so it is manufactured in large quantities. The...
Related questions
Question
100%
Can you help me solve this problem?
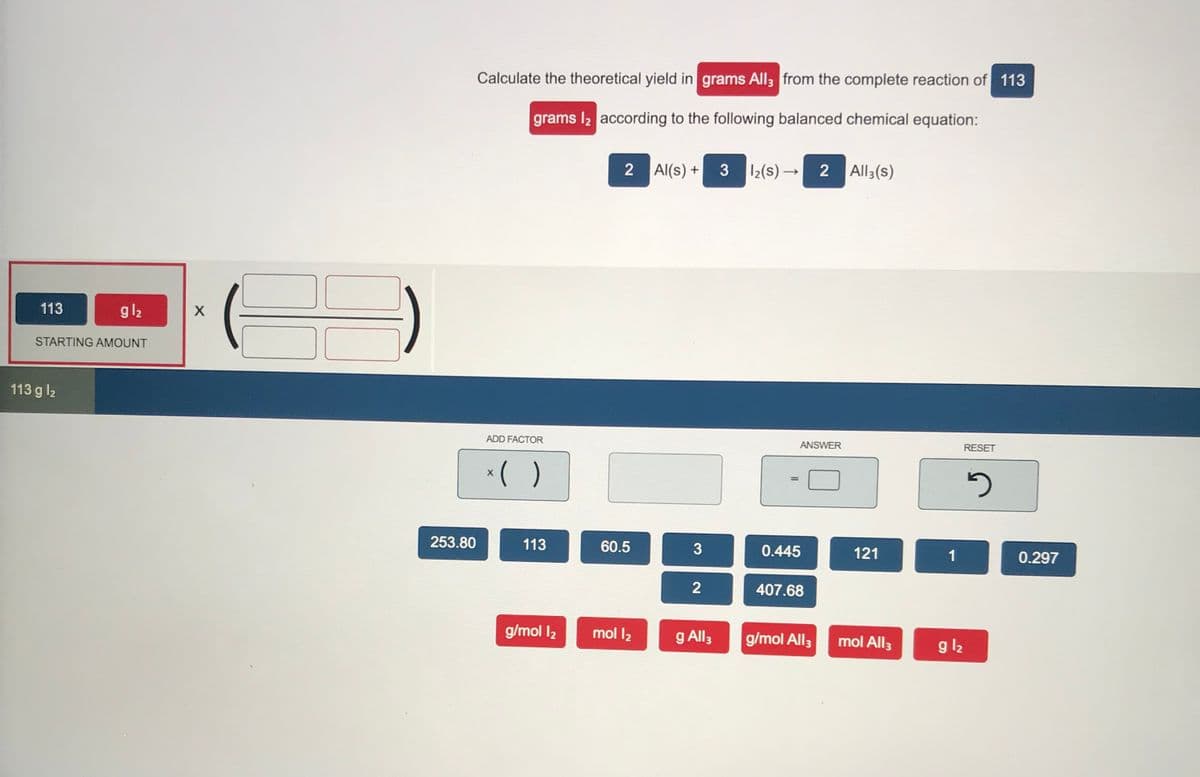
Transcribed Image Text:Calculate the theoretical yield in grams All3 from the complete reaction of 113
grams I2 according to the following balanced chemical equation:
2
Al(s) +
3
I2(s) →
2 All3(s)
113
gl2
STARTING AMOUNT
113 g l2
ADD FACTOR
ANSWER
RESET
()
X
%3D
253.80
113
60.5
0.445
121
1
0.297
2
407.68
g/mol 12
mol l2
g All3
g/mol All;
mol All3
g l2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
