Calculate the theoretical yield of silver chloride, if 5.15 g of neighborhood chloride have been added when silver nitrate is in excess. BaCl2 + 2AgNO3 --> 2AgCl + Ba(NO3)2
Q: 6. Consider the reaction: NO2(g) + CO(g) - NO(g) + CO₂(g) a. What would be the rate law for the…
A:
Q: b. The initial rate of the reaction was measured at several different concentrations of the…
A:
Q: what is the mass percent for O in so3
A:
Q: 2. Provide a name for each of the following. ð ð سکولاک z CI Br ongsão CH₂CH3 Br H CH(CH3)CH₂CH3 ""H
A:
Q: [Select] pairs of electrons. [Select] an anion. [Select] sea" of shared electrons. is the type of…
A: A chemical bond is an interaction between two atoms/ ions and involves electrons.
Q: 4) Trimethyl Aluminium exists as a: A)Dimer b) Monomer d) Hexamer e) Tetramer
A:
Q: Question 5 Analyse the 'H NMR spectrum of benzocaine below and complete the table of 'H NMR…
A: In the given problem we have to assign the protons , their ppm value, integration and multiplicity…
Q: How many moles of Argon are in 13.56L at STP? - 1.1 mol - 0.55 mol - 0.61 mol - 1.7 mol
A: Using the conditions of standard temperature and pressure and the given value of volume we can…
Q: Calculate ΔSϴ for the reduction of aluminum oxide by hydrogen gas: Al2O3(s) + 3H2(g) → 2Al(s) +…
A:
Q: Hydrogen gas and iodine react to form hydrogen iodide via the reaction H2(g)+I2(g)⇌2HI(g)…
A:
Q: For the following unbalanced redox reaction: Au + O₂ + CN™ [Au(CN)₂] + H₂O
A:
Q: Chemistry the unit is electro chemistry- grade 12:) How many H2O's must be added to the half…
A: According to the guidelines only one question is allowed to be solved at a time. I am answering 1st…
Q: 3. If liquid mercury has a contact angle, 9 of 140 with glass, which statement is correct? Liquid…
A: Cohesive force between the molecules of mercury are stronger than the adhesive force between the…
Q: (6.5)What is the molar volume of argon gas at STP? O 15.3 L 22.4 L O 4.00 L O 1.00 L
A: Find out the molar volume at stp
Q: The volume of a NaNO3 solution in 1000 g water at 25 °C and 1 atm can be described using the…
A:
Q: 2. SEP Develop a Model Use graph paper or graphing software to plot the moles of ammonia produced…
A: 2. We need to plot the graph of mol of NH3 produced (y axis) against moles of each reactants used (x…
Q: Briefly describe the activities that occur in each step of the drug discovery and development…
A: In the given question we need to describe in brief the activities that occur in each step of the…
Q: Which chemical has both ionic and covalent bonds? KNO3, HNO3, C6H12O6 How many p electrons are in…
A:
Q: Carbon dioxide (CO2) behaves as an ideal gas. A fixed quantity of this gas has a pressure of 5.6 x…
A: • The values provided in the question are:- i) Initial volume of CO2 gas, V1 = 1.53 L ii)…
Q: A 21.3 mL solution of HBr (a strong acid) of unknown concentration was titrated using 0.2735 mol L-1…
A:
Q: Solid phosphorus (P)and chlorine (C1₂) gas react to form solid phosphorus pentachloride (PCI).…
A: Mole can be defined as a standard unit for measuring large quantities of very small entities such as…
Q: (5.7: Similar to For practice 5.11)The titration of a 20.0 mL sample of an H₂SO4 solution of unknown…
A: H2SO4 is a diprotic acid with 2 removable protons(H+). KOH is a monoacidic base that can give 1…
Q: Answer the questions below using the lewis structure for IF3 provided. F: [Select] hybridization on…
A: Given that, Molecule= IF3 then,
Q: Convert 350 kg to ng
A: Here we are required to convert kg to ng.
Q: A + 2B C + 2D →>> The reaction is known to be first order in [A] and second order in [B]. When 0.30…
A:
Q: 23. Which species is the strongest oxidizing agent? 24. Which species is the strongest reducing…
A: The species that will oxidise other and itself reduces is called oxidizing agent Where the species…
Q: A mixture of gas contains 15% oxygen, 23% nitrogen, 4.0% argon and the rest carbon dioxide. If the…
A: The pressure of a gas depends on the number of moles of gas and it does not depend on the nature of…
Q: How many protons electrons and neurons does 56fe2+ have?
A:
Q: If I initially have a gas at a pressure of 12 atm, a volume of 23 litres, and a temperature of 200…
A:
Q: Q3/ Answer True or False for each of the following: 1) Salicylic acid can be prepared from…
A: Note! We can answer only first three subparts as per bartleby guidelines, kindly upload the rest…
Q: Identify the equilibrium below that shows the two chair conformations of the following compound: Br…
A: In the cyclohexane system, the most stable one is chair form. The equilibrium is set up between…
Q: The separation of APAP-L,(12 = 0.28 min) from APAP-D (W12 = 0.35 min) on a chiral stationary phase…
A: APAP-L W12 = 0.28 min has a retention time of 4.78 min and APAP-D W12 = 0.35 min has aretention…
Q: m b d Halogens Alkali Metals Alkali Earth Metals Noble Gases a. b. c. d. Inert. Do not react with…
A: The periodic table contains different elements arranged in the group and periods . The group one of…
Q: 1. What generalizations about an equilibrium constant can be made if the value for K is large? if…
A: if equilibrium constant (Keq) is large that means the concentration of products is more, that means…
Q: (5.9: Similar to Conceptual Connection 5.12) Which metal is most easily oxidized (with stronger…
A: Oxidation- Oxidation is the process of loss of electron Or gain of oxygen atom. Here in this case…
Q: Which electro O trigonal bipy O octahedral O tetrahedral O trigonal plana linear
A:
Q: What is the input material for the citric acid cycle, and other than energy carriers what are the…
A: Citric acid cycle or Kreb's cycle takes place inside the mitochondria matrix.
Q: An unmeasurable change in enthalpy can be calculated using the Hess law. The following is the…
A: We have to calculate the enthalpy change for following Reaction using Hess law - 2 CH4(g) ⇒…
Q: Calculate AG°for each of the following reactions from the equilibrium constant at the temperature…
A: Answer: Relation between equilibrium constant and standard Gibbs free energy change is shown below:…
Q: Question 1. (a) What is composite? Please write the name of few composites.
A: 1) (a) Composite is a a solid material that results when two or more different substances, each with…
Q: Generally, the larger the molar mass of a diatomic molecule, the O lower the viscosity O all of…
A: We know ; Larger the molar mass of the molecule larger will be the viscosity. Higher the molar mass…
Q: 9. Calculate the pressure differential of water across the surface of a spherical droplet of radius…
A: Surface tension is defined as the property of the surface of a liquid that allows it to resist an…
Q: do the current conditions in the gulf of maine support the growth and survivl of the soft shelled…
A:
Q: Chemistry Given the three-dimensional structure of the compound below determine the product of an E2…
A: Stereochemistry is branch of chemistry in which we deal with three dimensional arrangement of atoms…
Q: Consider the following reaction for the synthesis of nitrogen dioxide: N2(g) + 202(g) →→ 2NO(g)…
A: The given thermochemical equation is: N2(g) + 2O2(g) → 2NO2(g) ; ∆rHo = +34 kJ.mol-1
Q: How much will atmospheric carbon change in one year?10year and 100 years?
A: Solution - Atmospheric carbon - The concentration of carbon dioxide in Earth's atmosphere is…
Q: Consider the following system at equilibrium where AH° = 18.8 kJ/mol, and Kc = 9.52x10-², at 350 K.…
A:
Q: High fructose corn syrup is used industrially as a sweetener in everything from carbonated beverages…
A:
Q: An experiment studying the decomposition of NO2 gas at 383 °C generates the plot shown below with a…
A:
Q: Here is an isomer of 2,4-dimethylcyclihexonal. Complete the alternative chair confirmations on the…
A:
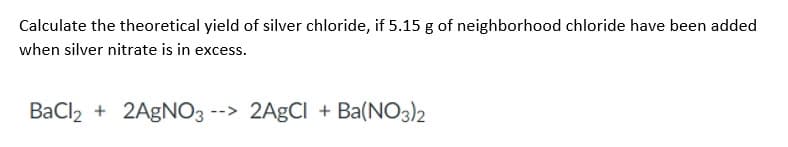
Step by step
Solved in 2 steps

- 1. How is complete precipitation assured in the Group III Cations? 2. In the confirmatory test of Magnesium ion, why is it necessary to heat the centrifugate in water bath for 5 minutes? 3. If Magnesium hydroxide is a white gelatinous precipitate, why is the confirmatory result indicated a blue precipitate?A 1.000-g sample containing bromide was dissolved in sufficient water to give 100.0 mL. A 50.00 mL aliquot was measured and after acidification, silver nitrate was introduced to precipitate AgBr, which was filtered, washed, and then dissolved in an ammoniacal solution of potassium tetracyanonickelate(II): Ni(CN)42- + 2AgBr(s) → 2Ag(CN)2- + Ni2+ + 2Br-50.00 mL remaining solution was analyzed for its Br- content by potentiometry using a metallic electrode of the second kind. a) Write the cell notation of the potentiometric set-up with SCE as the reference electrode. b) Write the Nernst equation that describes the indicator electrode set-up. Ecell recorded in running the solution using the potentiometric set-up was Ecell = 0.0286 V. (E0Ag/AgBr = 0.095 V) c) Compute for Eind. d) Compute pBr in the 50.00 mL aliquot. e) Compute for % NaBr ( in the potentiometric technique).Suppose that 0.323 g of an unknown sulfate salt is dissolved in 50 mL of water. The solution is acidified with 6 M HCl, heated, and an excess of aqueous BaCl2 is slowly added to the mixture resulting in the formation of a white precipitate. 1) Assuming that 0.433 g of precipitate is recovered calculate the percent by mass of SO42− in the unknown salt. 2) If it is assumed that the salt is an alkali sulfate determine the identity of the alkali cation.
- if we use KMnO4 and H3Po4 and MnSO4 for tritation of fecl3 and calculation of. Fe2+ what suitable ratio for tritationExplain why colour of KMnO4 disappears when oxalic acid is added to its solution in acidic medium.The digestion of a 0.1159 gram sample of a phosphorous-containing compound in a mixture of HNO3 and H2SO4 resulted in the formation of CO2, H2O, and H3PO4. Addition of ammonium molybdate yielded a solid having the composition (NH4)3PO4·12MoO3 (FW = 1876.3). This precipitate was filtered, washed, and dissolved in 50.00 mL of 0.2000 M NaOH: (NH4)3PO4•12MoO3(s)+26OH-(aq)->HPO42-(aq)+12MoO42-(aq)+14H2O(l)+3NH3(g) After the solution was boiled to remove the NH3, the excess NaOH was back-titrated with 14.84 mL of 0.1626 M HCl to a phenolphthalein end point. Calculate the percent phosphorous (FW = 30.9737) in the sample.
- Complete the balanced dissociation equation for the compound below in aqueous solution. AgI ->a) Which ion, Pt(II) or Mn(II), is more likely to form a sulfide in the presence of H2S in water. b) Rationalize your answer with the trends in hard and soft character. c) Give a balance chemical equation from your reaction.If the molar solubility of PbCrO4 at 25 oC is 5.48e-07 mol/L, what is the Ksp at this temperature?Ksp = (b) It is found that 1.08e-09 g of Fe(OH)3 dissolves per 100 mL of aqueous solution at 25 oC. Calculate the solubility-product constant for Fe(OH)3.Ksp = (c) The Ksp of Mg3(PO4)2 at 25 oC is 1.04e-24. What is the molar solubility of Mg3(PO4)2?solubility = mol/L
- A sample weighing 0.6760 g that contains an unknown amount of BaCl₂ was completely dissolved in water and treated with an excess of sodium sulfate. Na2SO4 A precipitate of BaSO4 formed which was dried and weighed, yielding 0.4105 g. What percentage of the original sample was BaCl₂? 39.77 24.15 35.72 69.78 54.18Assuming a bicarbonate ion concentration [HCO3-]of 1.00 × 10−3 M and a value of 3.5 × 10−11 for the solubility product of FeCO3, what would you expect to be the stable iron species at pH 9.5 and pE −8.0, as shown in Figure 3.4?You are assigned an unknown solution that contains Group III cations. To -1 mL of this solution was added 6 M NH3 the solution was agitated to mix well, and a reddish-brown precipitate with a gelatinous solid clinging to the inner walls of the test tube was observed. The solution was centrifuged and the supernatant was tested for completeness of precipitation by adding an additional drop of 6 M NH3. No cloudiness was observed as the drop of reagent diffused through the solution. The supernatant was then carefully decanted into a clean test tube, labeled (1st solution), and saved for further testing later. The precipitate remaining in the test tube was washed with a small amount of water, centrifuged, and the wash decanted and discarded. To the precipitate was added about 10 drops of 6 M NAOH plus ~1 mL H20 and the test tube was vigorously agitated. The resulting suspension was centrifuged and the supernatant liquid was transferred to another clean test tube and clearly labeled (2nd…

