Calculate the volume of 11.4 M HCI that must be used if you want to create 700 ml of 5.00 molar HCl. Round to 2 decimal places, include your units, and box your answer. Show Your Work show your work for all math problems, making sure that you round to 2 decimal places and don't forget the units. BOX YOUR FINAL ANSWER. Formulas: M1V1 = M2V2 Molarity = moles of solute/liters of solution %3D
Calculate the volume of 11.4 M HCI that must be used if you want to create 700 ml of 5.00 molar HCl. Round to 2 decimal places, include your units, and box your answer. Show Your Work show your work for all math problems, making sure that you round to 2 decimal places and don't forget the units. BOX YOUR FINAL ANSWER. Formulas: M1V1 = M2V2 Molarity = moles of solute/liters of solution %3D
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 4RQ: Molarity is a conversion factor relating moles of solute in solution to the volume of the solution....
Related questions
Question
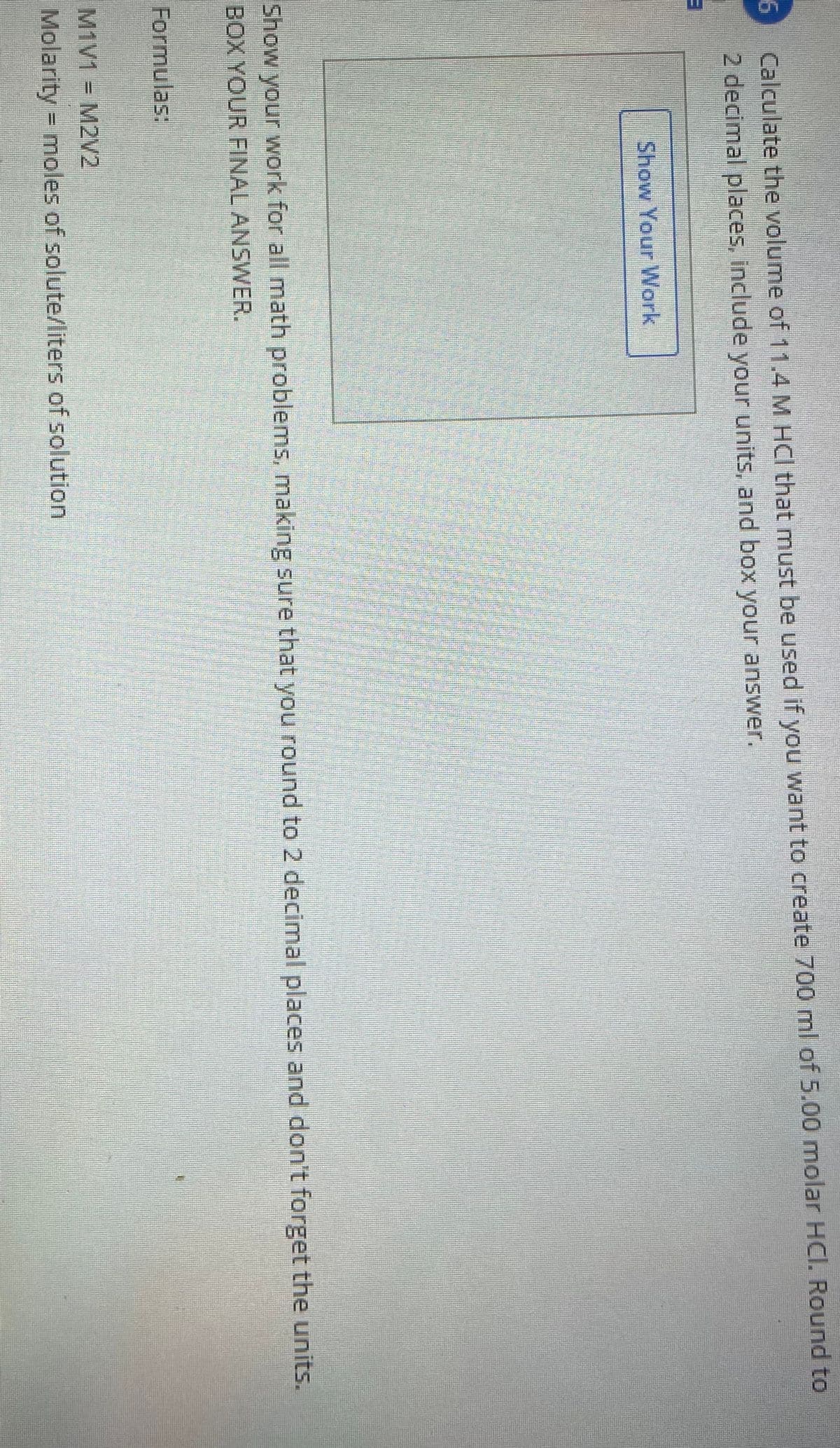
Transcribed Image Text:Calculate the volume of 11.4 M HCI that must be used if you want to create 700 ml of 5.00 molar HCI. Round to
2 decimal places, include your units, and box your answer.
Show Your Work
Show your work for all math problems, making sure that you round to 2 decimal places and don't forget the units.
BOX YOUR FINAL ANSWER.
Formulas:
M1V1
M2V2
=
Molarity = moles of solute/liters of solution
%D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning