Calculating an equilibrium constant from a heterogeneous equilibrium com... Calcium oxide and carbon dioxide react to form calcium carbonate, like this: CaO(s)+CO2(g)→ CaCO3(s) At a certain temperature, a chemist finds that a 5.6 L reaction vessel containing a mixture of calcium oxide, carbon dioxide, and calcium carbonate at equilibrium has the following composition: compound amount 10.8 g Cao CO 2 10.0 g CACO3 80.1 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K
Calculating an equilibrium constant from a heterogeneous equilibrium com... Calcium oxide and carbon dioxide react to form calcium carbonate, like this: CaO(s)+CO2(g)→ CaCO3(s) At a certain temperature, a chemist finds that a 5.6 L reaction vessel containing a mixture of calcium oxide, carbon dioxide, and calcium carbonate at equilibrium has the following composition: compound amount 10.8 g Cao CO 2 10.0 g CACO3 80.1 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.34PAE: 1’he reaction in Exercise 12.33 was repeated. This time, the reaction began when only NO was...
Related questions
Question
100%
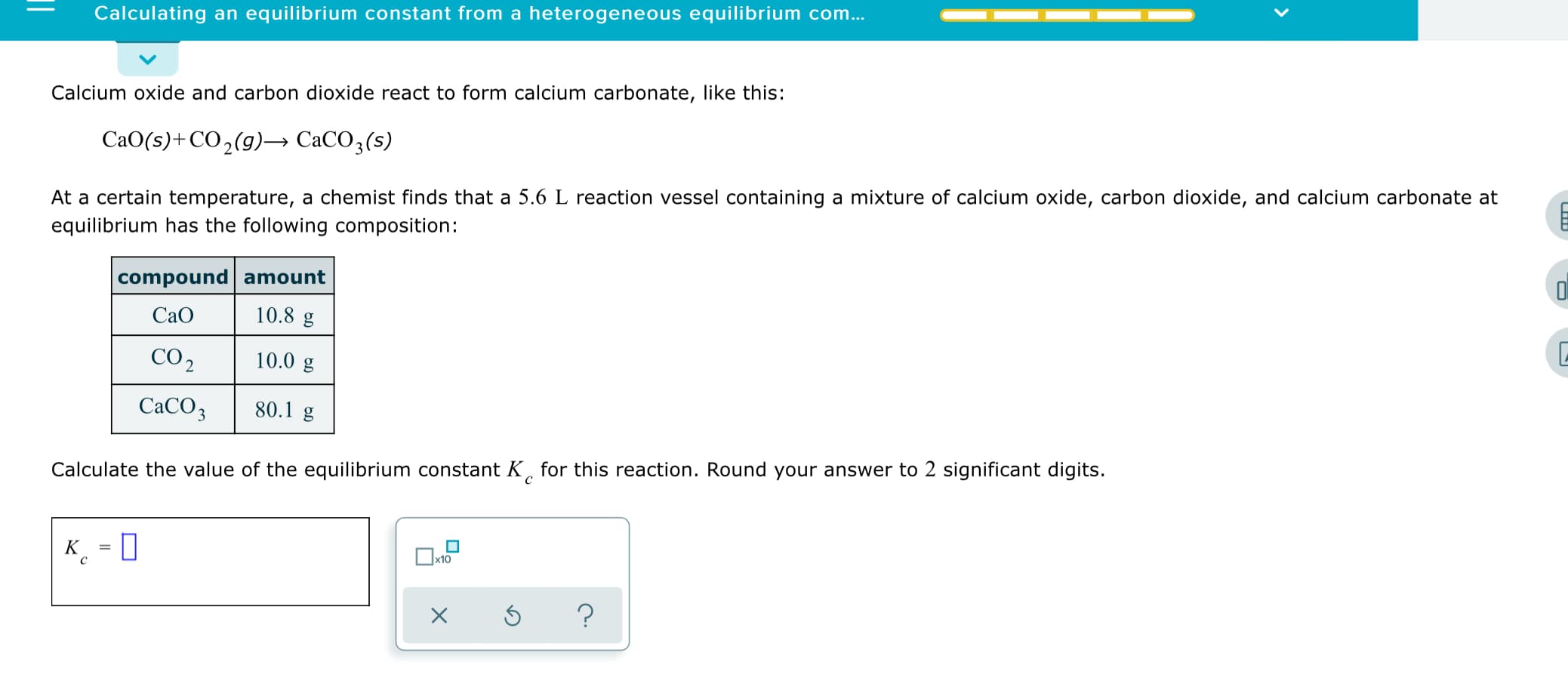
Transcribed Image Text:Calculating an equilibrium constant from a heterogeneous equilibrium com...
Calcium oxide and carbon dioxide react to form calcium carbonate, like this:
CaO(s)+CO2(g)→ CaCO3(s)
At a certain temperature, a chemist finds that a 5.6 L reaction vessel containing a mixture of calcium oxide, carbon dioxide, and calcium carbonate at
equilibrium has the following composition:
compound amount
10.8 g
Cao
CO 2
10.0 g
CACO3
80.1 g
Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits.
K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning