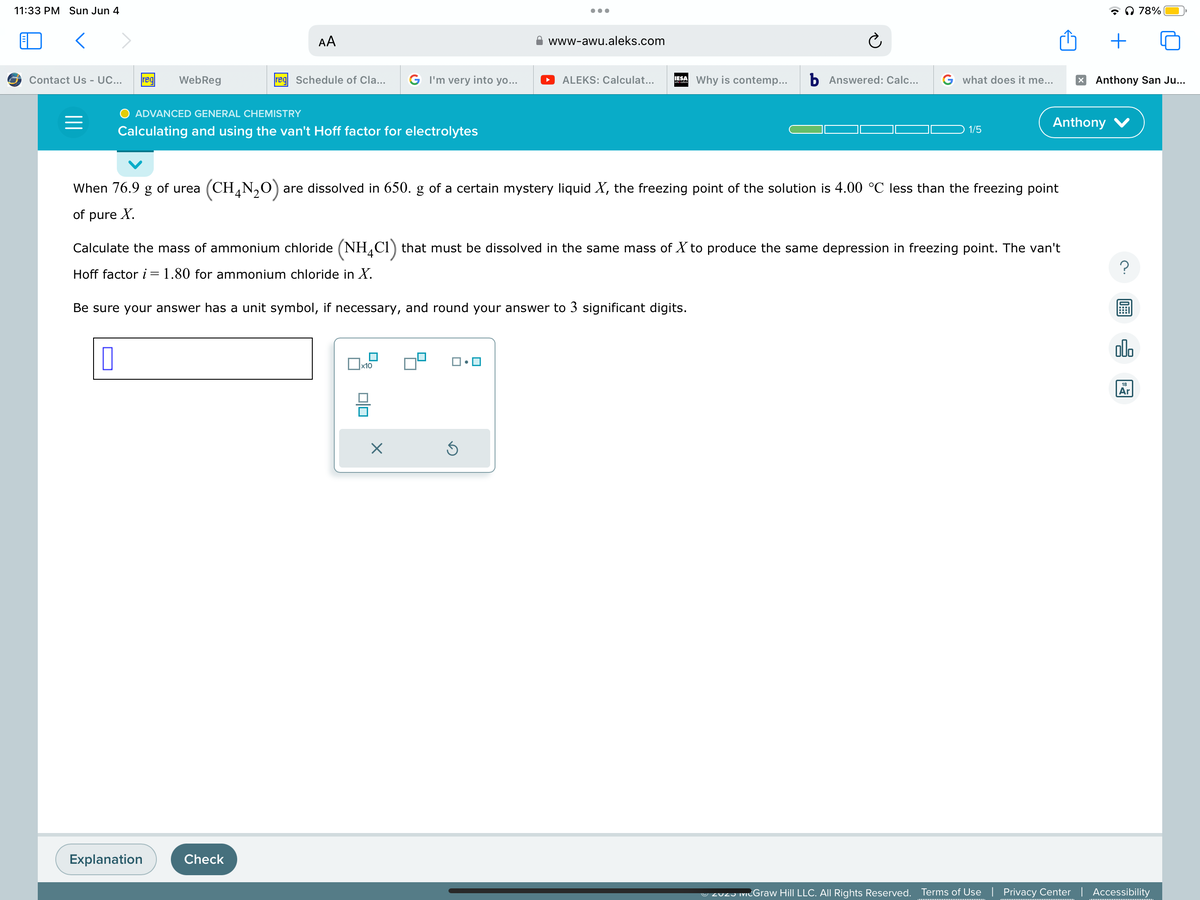
Calculating and using the van't Hoff factor for electrolytes When 76.9 g of urea (CH4N₂O) are dissolved in 650. g of a certain mystery liquid X, the freezing point of the solution is 4.00 °C less than the freezing point of pure X. Calculate the mass of ammonium chloride (NH4Cl) that must be dissolved in the same mass of X to produce the same depression in freezing point. The van't Hoff factor i = 1.80 for ammonium chloride in X. Be sure your answer has a unit symbol, if necessary, and round your answer to 3 significant digits. olo 0.0 ? 7 Ollo Ar
Calculating and using the van't Hoff factor for electrolytes When 76.9 g of urea (CH4N₂O) are dissolved in 650. g of a certain mystery liquid X, the freezing point of the solution is 4.00 °C less than the freezing point of pure X. Calculate the mass of ammonium chloride (NH4Cl) that must be dissolved in the same mass of X to produce the same depression in freezing point. The van't Hoff factor i = 1.80 for ammonium chloride in X. Be sure your answer has a unit symbol, if necessary, and round your answer to 3 significant digits. olo 0.0 ? 7 Ollo Ar
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.6PAE: At what points in the Chapman cycle do photochemical reactions take place?
Related questions
Question
Hello! Please keep in mind the sig figs it's asking for as well as the units if any. Thank you so much :)
Transcribed Image Text:11:33 PM Sun Jun 4
<
Contact Us - UC...
=
req
WebReg
0
AA
Explanation
req Schedule of Cla...
ADVANCED GENERAL CHEMISTRY
Calculating and using the van't Hoff factor for electrolytes
Check
G I'm very into yo...
x10
Be sure your answer has a unit symbol, if necessary, and round your answer to 3 significant digits.
010
When 76.9 g of urea (CHẠN,O) are dissolved in 650. g of a certain mystery liquid X, the freezing point of the solution is 4.00 °C less than the freezing point
of pure X.
X
●●●
Calculate the mass of ammonium chloride (NH4C1) that must be dissolved in the same mass of X to produce the same depression in freezing point. The van't
Hoff factor i = 1.80 for ammonium chloride in X.
www-awu.aleks.com
☐☐
ALEKS: Calculat...
5
IESA Why is contemp... b Answered: Calc...
G what does it me...
1/5
Anthony
+
X Anthony San Ju...
?
olo
78%
18
Ar
Zuz McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning