Carbon Dating The age of an ancient artifact can be deter- mined by the amount of radioactive carbon-14 remaining in it. If D, is the original amount of carbon-14 and D is the amount remaining, then the artifact's age A (in years) is given by A = -8267 In Find the age of an object if the amount D of carbon-14 that remains in the object is 73% of the original amount D..
Carbon Dating The age of an ancient artifact can be deter- mined by the amount of radioactive carbon-14 remaining in it. If D, is the original amount of carbon-14 and D is the amount remaining, then the artifact's age A (in years) is given by A = -8267 In Find the age of an object if the amount D of carbon-14 that remains in the object is 73% of the original amount D..
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter10: Radioactivity And Nuclear Processes
Section: Chapter Questions
Problem 10.1E
Related questions
Question
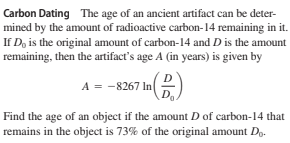
Transcribed Image Text:Carbon Dating The age of an ancient artifact can be deter-
mined by the amount of radioactive carbon-14 remaining in it.
If D, is the original amount of carbon-14 and D is the amount
remaining, then the artifact's age A (in years) is given by
A = -8267 In
Find the age of an object if the amount D of carbon-14 that
remains in the object is 73% of the original amount D..
Expert Solution
Step 1
Radioactive decay follows first order kinetics. The integrated rate equation for a first order reaction is
Case 1:
Initial concentration = Do
Final concentration = D
Time = A years
The integrated rate equation is given as
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning