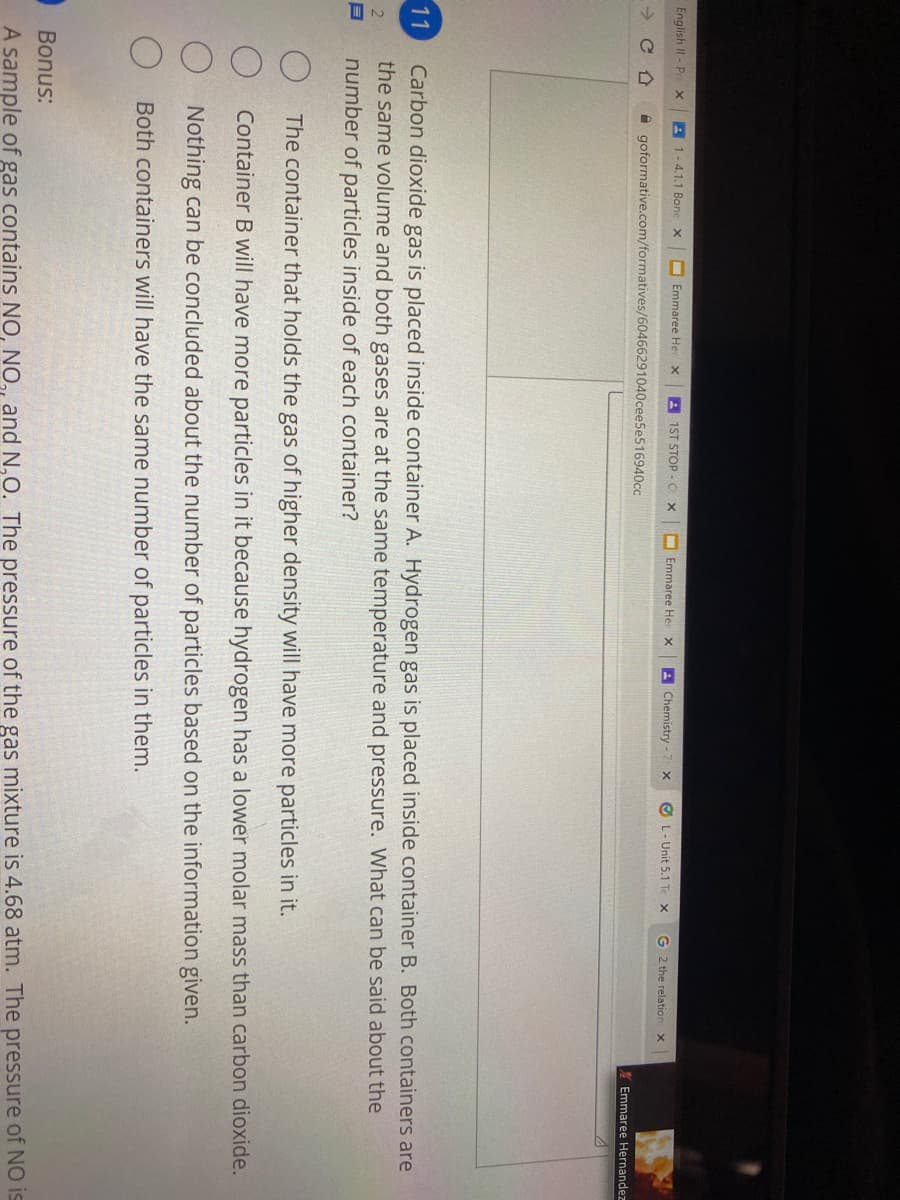
Carbon dioxide gas is placed inside container A. Hydrogen gas is placed inside container B. Both containers are the same volume and both gases are at the same temperature and pressure. What can be said about the number of particles inside of each container? O The container that holds the gas of higher density will have more particles in it. O Container B will have more particles in it because hydrogen has a lower molar mass than carbon dioxide. Nothing can be concluded about the number of particles based on the information given. Both containers will have the same number of particles in them.
Carbon dioxide gas is placed inside container A. Hydrogen gas is placed inside container B. Both containers are the same volume and both gases are at the same temperature and pressure. What can be said about the number of particles inside of each container? O The container that holds the gas of higher density will have more particles in it. O Container B will have more particles in it because hydrogen has a lower molar mass than carbon dioxide. Nothing can be concluded about the number of particles based on the information given. Both containers will have the same number of particles in them.
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 7P
Related questions
Question
Transcribed Image Text:English II- Pre x
A 1-4.1.1 Bone x
I Emmaree Her
A 1ST STOP-CX
I Emmaree Her x
A Chemistry
O L-Unit 5.1 Te X
G 2 the relation
A goformative.com/formatives/60466291040cee5e516940cc
Emmaree Hernandez
Carbon dioxide gas is placed inside container A. Hydrogen gas is placed inside container B. Both containers are
the same volume and both gases are at the same temperature and pressure. What can be said about the
number of particles inside of each container?
11
The container that holds the gas of higher density will have more particles in it.
Container B will have more particles in it because hydrogen has a lower molar mass than carbon dioxide.
Nothing can be concluded about the number of particles based on the information given.
Both containers will have the same number of particles in them.
Bonus:
A sample of gas contains NO, NO,, and N,O. The pressure of the gas mixture is 4.68 atm. The pressure of NO is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning