Carbon is an interstitial atom in Alloy B, and may occupy any of the following positions in the iron lattice: (½,0,0); (0,½,0); (0,%½,1) and (½,1,0). a) With the aid of a clearly labelled diagram, describe an interstitial atom. b) Explain how carbon increases the strength of iron. c) Draw a unit cell to show a carbon atom at position (0,½,1).
Carbon is an interstitial atom in Alloy B, and may occupy any of the following positions in the iron lattice: (½,0,0); (0,½,0); (0,%½,1) and (½,1,0). a) With the aid of a clearly labelled diagram, describe an interstitial atom. b) Explain how carbon increases the strength of iron. c) Draw a unit cell to show a carbon atom at position (0,½,1).
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
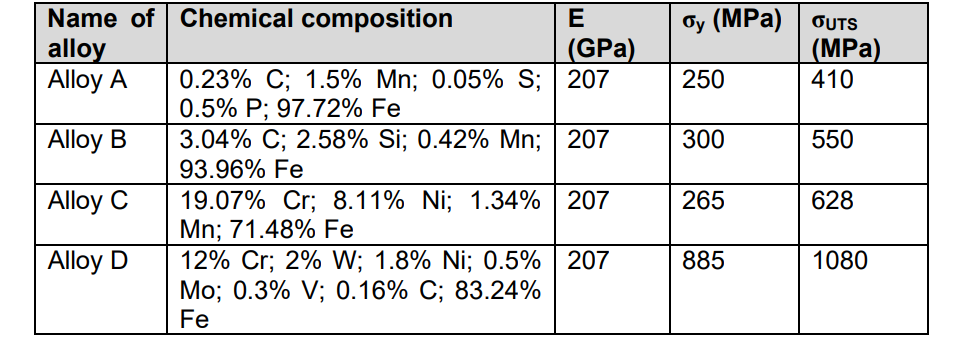
Transcribed Image Text:Name of Chemical composition
alloy
Alloy A
E
бy (MPa) | OUTS
(МPа)
410
(GPa)
0.23% C; 1.5% Mn; 0.05% S; | 207
0.5% P; 97.72% Fe
3.04% C; 2.58% Si; 0.42% Mn; | 207
93.96% Fe
250
Alloy B
300
550
19.07% Cr; 8.11% Ni; 1.34% 207
Mn; 71.48% Fe
12% Cr; 2% W; 1.8% Ni; 0.5% 207
Mo; 0.3% V; 0.16% C; 83.24%
Fe
Alloy C
265
628
Alloy D
885
1080

Transcribed Image Text:2.2.
Carbon is an interstitial atom in Alloy B, and may occupy any of the following
positions in the iron lattice: (%,0,0); (0,½,0); (0,½,1) and (½,1,0).
a) With the aid of a clearly labelled diagram, describe an interstitial atom.
b) Explain how carbon increases the strength of iron.
c) Draw a unit cell to show a carbon atom at position (0,½,1).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY