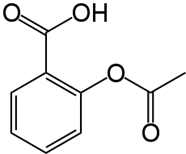
Case Study Section 1 – pH Based on “The Case of the Mortified Mom: Acids, pH and Buffers” by Terry Platt (National Center for Case Study Teaching in Science) The Patient: Paramedics were called to the home of the Mathews family because their 3-year old daughter, Molly, had gotten into the medicine cabinet and consumed a large number of aspirin tablets. When the paramedics arrived Molly had vomited several times, with bits of undissolved tablets visible, but seemed sleepy, almost lethargic. She was rushed to the nearest Emergency Room. When she reached the hospital she was unarousable and was breathing rapidly and deeply. She was examined and lab samples were obtained. Above is the structure of aspirin, or acetylsalicylic acid, which is a weak acid with a pKa of 3.5. The active, and toxic at high doses, form is the protonated form. Question 9: What would be the consequence(s) of lower than normal blood pH? Cause proteins to denature Decrease in enzyme activities Limit hemoglobin’s ability to transport oxygen All of the above None of the above
Ionic Equilibrium
Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Ionic equilibrium is established between the ions and unionized species in a system. Understanding the concept of ionic equilibrium is very important to answer the questions related to certain chemical reactions in chemistry.
Arrhenius Acid
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red.
Bronsted Lowry Base In Inorganic Chemistry
Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with.
Case Study Section 1 – pH
Based on “The Case of the Mortified Mom: Acids, pH and Buffers” by Terry Platt (National Center for Case Study Teaching in Science)
The Patient:
Paramedics were called to the home of the Mathews family because their 3-year old daughter, Molly, had gotten into the medicine cabinet and consumed a large number of aspirin tablets. When the paramedics arrived Molly had vomited several times, with bits of undissolved tablets visible, but seemed sleepy, almost lethargic. She was rushed to the nearest Emergency Room. When she reached the hospital she was unarousable and was breathing rapidly and deeply. She was examined and lab samples were obtained.
Above is the structure of aspirin, or acetylsalicylic acid, which is a weak acid with a pKa of 3.5. The active, and toxic at high doses, form is the protonated form.
Question 9:
What would be the consequence(s) of lower than normal blood pH?
- Cause proteins to denature
- Decrease in enzyme activities
- Limit hemoglobin’s ability to transport oxygen
- All of the above
- None of the above
Trending now
This is a popular solution!
Step by step
Solved in 2 steps


