cation Ca²+ 3+ Mn 2+ Ba Cu anion NO₂ 104 BrO PO some ionic compounds empirical formula Ca(NO₂)₂ Mn (104) Ba (BrO3)₂ Cu₂PO4 name of compound calcium(II) nitrite Manganese(III) periodate barium(II) bromate copper(I) phosphate 4 X
cation Ca²+ 3+ Mn 2+ Ba Cu anion NO₂ 104 BrO PO some ionic compounds empirical formula Ca(NO₂)₂ Mn (104) Ba (BrO3)₂ Cu₂PO4 name of compound calcium(II) nitrite Manganese(III) periodate barium(II) bromate copper(I) phosphate 4 X
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter5: Nomenclature
Section: Chapter Questions
Problem 14CR
Related questions
Question
Transcribed Image Text:1 ain
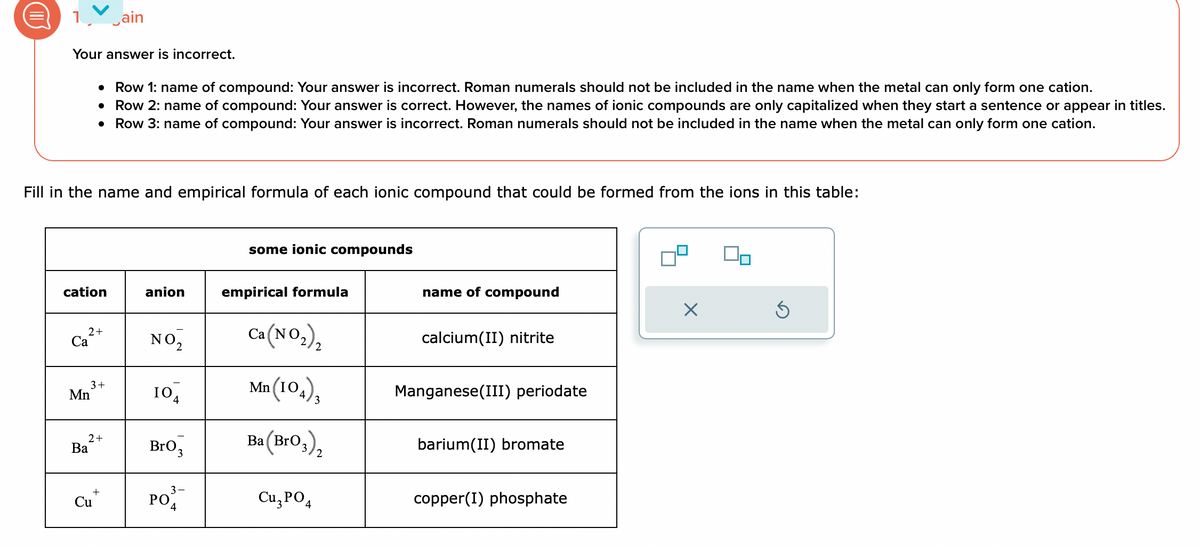
Your answer is incorrect.
Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table:
cation
2 +
Ca
• Row 1: name of compound: Your answer is incorrect. Roman numerals should not be included in the name when the metal can only form one cation.
●
Row 2: name of compound: Your answer is correct. However, the names of ionic compounds are only capitalized when they start a sentence or appear in titles.
• Row 3: name of compound: Your answer is incorrect. Roman numerals should not be included in the name when the metal can only form one cation.
Mn
3+
Ba
2+
+
Cu
anion
NO₂
10
BrO 3
3
PO
some ionic compounds
empirical formula
Ca(NO₂)₂
Mn (104),
3
Ba (BrO3)₂
Cu3PO4
name of compound
calcium(II) nitrite
Manganese(III) periodate
barium(II) bromate
copper(I) phosphate
X
00
S
Expert Solution
Step 1
We can write the names of ionic compounds from their cations and anions.
We write cation first and then anion. And if the metal can show different valency, then we need to mention the oxidation state of metal.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning