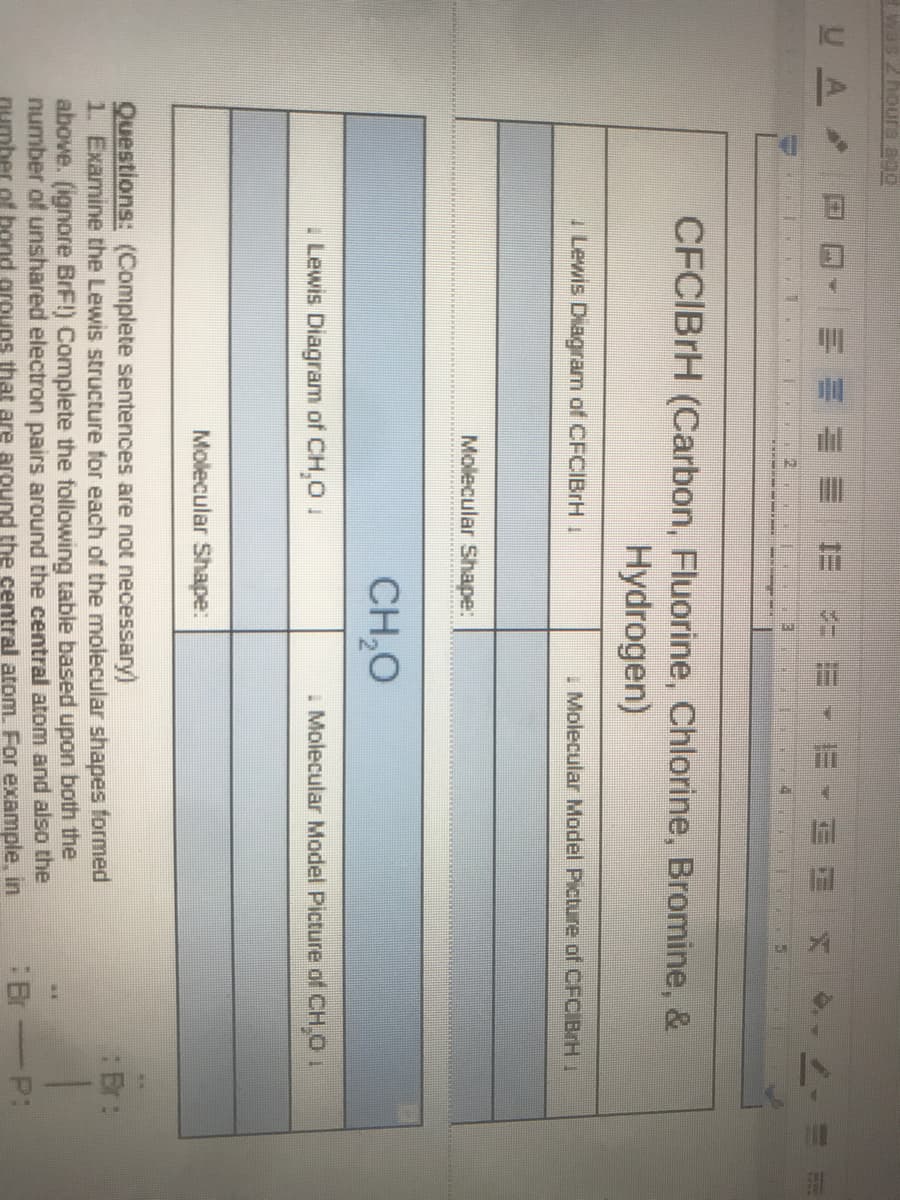
CFCIBrH (Carbon, Fluorine, Chlorine, Bromine, & Hydrogen) I Lewis Diagram of CFCIBrH I I Molecular Model Picture of CFCIBrH 1 Molecular Shape: CH,O . Lewis Diagram of CH,0 1 I Molecular Model Picture of CH.O I Molecular Shape:
CFCIBrH (Carbon, Fluorine, Chlorine, Bromine, & Hydrogen) I Lewis Diagram of CFCIBrH I I Molecular Model Picture of CFCIBrH 1 Molecular Shape: CH,O . Lewis Diagram of CH,0 1 I Molecular Model Picture of CH.O I Molecular Shape:
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter4: Molecular Structure And Orbitals
Section: Chapter Questions
Problem 3RQ: Consider the following compounds: CO2, SO2, KrF2, SO3, NF3, IF3, CF4, SF4, XeF4, PF5, TF5, and SCl6....
Related questions
Question
Draw a picture for each section, molecular model picture and lewis diagram and molecular shape please
Transcribed Image Text:= 1三
CFCIBRH (Carbon, Fluorine, Chlorine, Bromine, &
Hydrogen)
I Lewis Diagram of CFCIBrH I
I Molecular Model Picture of CFCIBrH 1
Molecular Shape:
CH,0
. Lewis Diagram of CH,0 1
I Molecular Model Picture of CH.O 1
Molecular Shape:
Questions: (Complete sentences are not necessary)
1. Examine the Lewis structure for each of the molecular shapes formed
above. (ignore BrF!) Complete the following table based upon both the
number of unshared electron pairs around the central atom and also the
number of bond groups that are around the central atom. For example, in
Er:
:Br
P:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning