Chemistry 10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
1 Chemical Foundations 2 Atoms, Molecules, And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding: General Concepts 9 Covalent Bonding: Orbitals 10 Liquids And Solids 11 Properties Of Solutions 12 Chemical Kinetics 13 Chemical Equilibrium 14 Acids And Bases 15 Acid-base Equilibria 16 Solubility And Complex Ion Equilibria 17 Spontaneity, Entropy, And Free Energy 18 Electrochemistry 19 The Nucleus: A Chemist's View 20 The Representative Elements 21 Transition Metals And Coordination Chemistry 22 Organic And Biological Molecules Chapter6: Thermochemistry
Chapter Questions Section: Chapter Questions
Problem 1RQ: Define the following terms: potential energy, kinetic energy, path-dependent function, state... Problem 2RQ: Consider the following potential energy diagrams for two different reactions. Which plot represents... Problem 3RQ: What is the first law of thermodynamics? How can a system change its internal energy, E? What are... Problem 4RQ: When a gas expands, what is the sign of w? Why? When a gas contracts, what is the sign of w? Why?... Problem 5RQ: What is the heat gained/released at constant pressure equal1o (qp = ?)? What is the heat... Problem 6RQ: High-quality audio amplifiers generate large amounts of heat. To dissipate the heat and prevent... Problem 7RQ: Explain how calorimetry works to calculate H or E for a reaction. Does the temperature of the... Problem 8RQ: What is Hesss law? When a reaction is reversed, what happens to the sign and magnitude of H for that... Problem 9RQ: Define the standard enthalpy of formation. What are standard states for elements and for compounds?... Problem 1ALQ: Objects placed together eventually reach the same temperature. When you go into a room and touch a... Problem 2ALQ: What is meant by the term lower in energy? Which is lower in energy, a mixture of hydrogen and... Problem 3ALQ: A fire is started in a fireplace by striking a match and lighting crumpled paper under some logs.... Problem 4ALQ: Liquid water turns to ice. Is this process endothermic or exothermic? Explain what is occurring... Problem 5ALQ: Consider the following statements: ''Heat is a form of energy, and energy is conserved. The heat... Problem 6ALQ: Consider 5.5 L of a gas at a pressure of 3.0 atm in a cylinder with a movable piston. The external... Problem 7ALQ: Consider 5.5 L of a gas at a pressure of 3.0 atm in a cylinder with a movable piston. The external... Problem 8ALQ: What if energy was not conserved? How would this affect our lives? Problem 9ALQ: Hesss law is really just another statement of the first law of thermodynamics. Explain. Problem 10ALQ: In the equation w = P V, why is there a negative sign? Problem 13Q: Consider an airplane trip from Chicago, Illinois, to Denver, Colorado. List some path-dependent... Problem 14Q: How is average bond strength related to relative potential energies of the reactants and the... Problem 15Q: Assuming gasoline is pure C8H18(l), predict the signs of q and w for the process of combusting... Problem 16Q: What is the difference between H and E? Problem 17Q: The enthalpy change for the reaction CH4(g)+2O2(g)CO2(g)+2H2O(l) is 891 kJ for the reaction as... Problem 18Q: Explain why oceanfront areas generally have smaller temperature fluctuations than inland areas. Problem 19Q: The equation for the fermentation of glucose to alcohol and carbon dioxide is: C6H12O6(aq) ... Problem 20Q: Explain why H is obtained directly from coffee-cup calorimeters, whereas E is obtained directly from... Problem 21Q: The enthalpy of combustion of CH4(g) when H2O (1) is formed is 891 kJ/mol and the enthalpy of... Problem 22Q: The enthalpy change for a reaction is a state function and it is an extensive property. Explain. Problem 23Q: Standard enthalpies of formation are relative values. What are Hfvalues relative to? Problem 24Q: The combustion of methane can be represented as follows: a. Use the information given above to... Problem 25Q: Why is it a good idea to rinse your thermos bottle with hot water before filling it with hot coffee? Problem 26Q Problem 27Q: What is incomplete combustion of fossil fuels? Why can this be a problem? Problem 28Q: Explain the advantages and disadvantages of hydrogen as an alternative fuel. Problem 29E Problem 30E: Which has the greater kinetic energy, an object with a mass of 2.0 kg and a velocity of 1.0 m/s or... Problem 31E: Consider the following diagram when answering the questions below. a. Compare balls A and B in terms... Problem 33E: A gas absorbs 45 kJ of heat and does 29 kJ of work. Calculate E. Problem 34E: Calculate E for each of the following. a. q = 47 kJ, w = + 88 kJ b. q = +82 kJ, w = 47 kJ c. q = +... Problem 35E: A system undergoes a process consisting of the following two steps: Step 1: The system absorbs 72 J... Problem 37E: If the internal energy of a thermodynamic system is increased by 300. J while 75 J of expansion work... Problem 38E: Calculate the internal energy change for each of the following. a. One hundred (100.) joules of work... Problem 39E: A sample of an ideal gas at 15.0 atm and 10.0 L is allowed to expand against a constant external... Problem 40E: A piston performs work of 210. L atm on the surroundings, while the cylinder in which it is placed... Problem 41E: Consider a mixture of air and gasoline vapor in a cylinder with a piston. The original volume is 40.... Problem 42E: As a system increases in volume, it absorbs 52.5 J of energy in the form of heat from the... Problem 43E: A balloon filled with 39.1 moles of helium has a volume of 876 L at 0.0C and 1.00 atm pressure. The... Problem 44E: One mole of H2O(g) at 1.00 atm and 100.C occupies a volume of 30.6 L. When 1 mole of H2O(g) is... Problem 45E: One of the components of polluted air is NO. It is formed in the high-temperature environment of... Problem 46E: The reaction SO3(g)+H2O(l)H2SO4(aq) is the last step in the commercial production of sulfuric acid.... Problem 47E: Are the following processes exothermic or endothermic? a. When solid KBr is dissolved in water, the... Problem 48E: Are the following processes exothermic or endothermic? a. the combustion of gasoline in a car engine... Problem 49E: The overall reaction in a commercial heat pack can be represented as 4Fe(s)+3O2(g)2Fe2O3(s)H=1652KJ... Problem 50E: Consider the following reaction: 2H2(g)+O2(g)2H2O(l)H=572KJ a. How much heat is evolved for the... Problem 51E: Consider the combustion of propane: C3H8(g)+5O2(g)3CO2(g)+4H2O(l)H=2221KJ Assume that all the heat... Problem 52E: Consider the following reaction: CH4(g)+2O2(g)CO2(g)+2H2O(l)H=891KJ Calculate the enthalpy change... Problem 53E: For the process H2O(l) H2O(g) at 298 K and 1.0 atm. H is more positive than E by 2.5 kJ/mol. What... Problem 54E: For the following reactions at constant pressure, predict if H E, H E, or H = E. a. 2HF(g) H2(g)... Problem 55E: Consider the substances in Table 7-1. Which substance requires the largest amount of energy to raise... Problem 56E: The specific heat capacity of silver is 0.24 J/Cg. a. Calculate the energy required to raise the... Problem 57E: A 500-g sample of one of the substances listed in Table 7-1 was heated from 25.2C to 55.1C,... Problem 58E: It takes 585 J of energy to raise the temperature of 125.6 g mercury from 20.0C to 53.5C. Calculate... Problem 59E: A 30.0-g sample of water at 280. K is mixed with 50.0 g water at 330. K. Calculate the final... Problem 60E: A biology experiment requires the preparation of a water bath at 37.0C (body temperature). The... Problem 61E: A 5.00-g sample of aluminum pellets (specific heat capacity = 0.89 J/C g) and a 10.00-g sample of... Problem 62E: Hydrogen gives off 120. J/g of energy when burned in oxygen, and methane gives off 50. J/g under the... Problem 63E: A 150.0-g sample of a metal at75.0C is added to 150.0 g H2O at 15.0C. The temperature of the water... Problem 64E: A 110.-g sample of copper (specific heat capacity = 0.20 J/C g) is heated to 82.4C and then placed... Problem 65E: In a coffee-cup calorimeter, 50.0 mL of 0.100 M AgNO3 and 50.0 mL of 0.100 M HCl are mixed to yield... Problem 66E: In a coffee-cup calorimeter, 100.0 mL of 1.0 M NaOH and 100.0 mL of 1.0 M HCI are mixed. Both... Problem 67E: A coffee-cup calorimeter initially contains 125 g water at 24.2C. Potassium bromide (10.5 g), also... Problem 68E: In a coffee-cup calorimeter, 1.60 g NH4NO3 is mixed with 75.0 g water at an initial temperature of... Problem 69E: Consider the dissolution of CaCl2: CaCl2(s)Ca2+(aq)+2Cl(aq)H=81.5KJ An 11.0-g sample of CaCl2 is... Problem 70E: Consider the reaction 2HCl(aq)+Ba(OH)2(aq)BaCl2(aq)+2H2O(l)H=118KJ Calculate the heat when 100.0 rnL... Problem 71E: Quinone is an important type of molecule that is involved in photosynthesis. The transport of... Problem 72E: The energy content of food is typically determined using a bomb calorimeter. Consider the combustion... Problem 73E: The heat capacity of a bomb calorimeter was determined by burning 6.79 g methane (energy of... Problem 74E: The combustion of 0.1584 g benzoic acid increases the temperature of a bomb calorimeter by 2.54C.... Problem 75E: The enthalpy of combustion of solid carbon to form carbon dioxide is 393.7 KJ/mol carbon, and the... Problem 76E: Combustion reactions involve reacting a substance with oxygen. When compounds containing carbon and... Problem 77E: Given the following data calculate H for the reaction On the basis of the enthalpy change, is this a... Problem 78E Problem 79E: The bombardier beetle uses an explosive discharge as a defensive measure. The chemical reaction... Problem 80E: Calculate H for the reaction 2NH3(g)+12O2(g)N2H4(l)+H2O(l) given the following data:... Problem 81E: Given the following data... Problem 82E: Given the following data... Problem 83E: Give the definition of the standard enthalpy of formation for a substance. Write separate reactions... Problem 84E: Write reactions for which the enthalpy change will be a. Hf for solid aluminum oxide. b. the... Problem 85E: Use the values ofHf in Appendix 4 to calculate H for the following reactions. a. b.... Problem 86E: Use the values of Hf in Appendix 4 to calculate H for the following reactions. (See Exercise 77 .)... Problem 87E: The Ostwald process for the commercial production of nitric acid from ammonia and oxygen involves... Problem 88E: Calculate H for each of the following reactions using the data in Appendix 4:... Problem 89E: The reusable booster rockets of the space shuttle use a mixture of aluminum and ammonium perchlorate... Problem 90E: The space shuttle Orbiter utilizes the oxidation of methylhydrazine by dinitrogen tetroxide for... Problem 91E: Consider the reaction 2ClF3(g)+2NH3(g)N2(g)+6HF(g)+Cl2(g)H=1196KJ Calculate H for CIF3(g). Problem 92E: The standard enthalpy of combustion of ethene gas, C2H4(g), is 1411.1 kJ/mol at 298 K. Given the... Problem 93E: Water gas is produced from the reaction of steam with coal: C(s)+H2O(g)H2(g)+CO(g) Assuming that... Problem 94E: Syngas can be burned directly or converted to methanol. Calculate H for the reaction... Problem 95E: Ethanol (C2H5OH) has been proposed as an alternative fuel. Calculate the standard enthalpy of... Problem 96E: Methanol (CH3OH) has also been proposed as an alternative fuel. Calculate the standard enthalpy of... Problem 97E: Some automobiles and buses have been equipped to bum propane (C3H8). Compare the amounts of energy... Problem 98E: Acetylene (C2H2) and butane (C4H10) are gaseous fuels with enthalpies of combustion of 49.9 kJ/g and... Problem 99E Problem 100E: The complete combustion of acetylene, C2H2(g), produces 1300. kJ of energy per mole of acetylene... Problem 101AE: It has been determined that the body can generate 5500 kJ of energy during one hour of strenuous... Problem 102AE: One way to lose weight is to exercise! Walking briskly at 4.0 miles per hour for an hour consumes... Problem 103AE: Three gas-phase reactions were run in a constant-pressure piston apparatus as shown in the following... Problem 104AE: Nitrogen gas reacts with hydrogen gas to form ammonia gas .Consider the reaction between nitrogen... Problem 105AE: Combustion of table sugar produces CO2(g) and H2O( l). When 1.46 g table sugar is combusted in a... Problem 106AE Problem 107AE: A serving size of six cookies contains 4 g of fat, 20 g of carbohydrates, and 2 g of protein. If... Problem 108AE: Calculate H for the reaction 2K(s)+2H2O(l)2KOH(aq)+H2(g) A 5.00-g chunk of potassium is dropped into... Problem 109AE: The enthalpy of neutralization for the reaction of a strong acid with a strong base is 56 kJ/mol... Problem 110AE: Given the following data: NO2(g) NO(g) + O(g)H = 233 kJ 2O3(g) 3O2(g)H = 427 kJ NO(g) + O3(g) ... Problem 111AE: If a student performs an endothermic reaction in a calorimeter, how does the calculated value of H... Problem 112AE: In a bomb calorimeter, the reaction vessel is surrounded by water that must be added for each... Problem 113AE: The bomb calorimeter in Exercise 102 is filled with 987 g water. The initial temperature of the... Problem 114AE: Consider the two space shuttle fuel reactions in Exercises 81 and 82. Which reaction produces more... Problem 115AE: Consider the following equations: 3A+6B3DH=403KJ/molE+2FAH=105.2KJ/molCE+3DH=64.8KJ/mol Suppose the... Problem 116AE: Given the following data... Problem 117AE: At 298 K, the standard enthalpies of formation for C2H2(g) and C6H6(l) are 227 kJ/mol and 49 kJ/mol,... Problem 118AE: Using the following data, calculate the standard heat of formation of ICl(g) in kJ/mol:... Problem 119AE: A sample of nickel is heated to 99.8C and placed in a coffee-cup calorimeter containing 150.0 g... Problem 120AE: Given: 2Cu2O(s) + O2(g) 4CuO(s)H = 288 kJ Cu2O(s) CuO(s) + CuO(s)H = 11kJ Calculate the standard... Problem 121AE: Calculate H for each of the following reactions, which occur in the atmosphere. a. C2H4(g) + O3(g) ... Problem 122CWP: Consider a balloon filled with helium at the following conditions. 313 g He 1.00 atm 1910. L Molar... Problem 123CWP: In which of the following systems is(are) work done by the surroundings on the system? Assume... Problem 124CWP: Which of the following processes are exothermic? a. N2(g)2N(g) b. H2O(l)H2O(s) c. Cl2(g)2Cl(g) d.... Problem 125CWP: Consider the reaction B2H6(g)+3O2(s)B2O3(s)+3H2O(g)H=2035KJ Calculate the amount of heat released... Problem 126CWP: A swimming pool, 10.0 m by 4.0 m, is filled with water to a depth of 3.0 m at a temperature of... Problem 127CWP: In a coffee-cup calorimeter, 150.0 mL of 0.50 M HCI is added to 50.0 mL of 1.00 M NaOH to make 200.0... Problem 128CWP: Calculate H for the reaction N2H4(l)+O2(g)N2(g)+2H2O(l) given the following data: Equation H(KJ)... Problem 129CWP: Which of the following substances have an enthalpy of formation equal to zero? a. Cl2(g) b. H2(g) c.... Problem 130CP: Consider 2.00 moles of an ideal gas that are taken from state A (PA = 2.00 atm, vA = 10.0 L) to... Problem 131CP: For the process H2O(l)H2O(g) at 298 K and 1.0 atm, H is more positive than E by 2.5 kJ/mol. What... Problem 132CP: The sun supplies energy at a rate of about 1.0 kilowatt per square meter of surface area (1 watt = 1... Problem 133CP: The best solar panels currently available are about 15% efficient in converting sunlight to... Problem 134CP: On Easter Sunday, April 3, 1983, nitric acid spilled from a tank car near downtown Denver, Colorado.... Problem 135CP: A piece of chocolate cake contains about 400 calories. A nutritional calorie is equal to 1000... Problem 136CP: The standard enthalpies of formation for S(g), F(g), SF4(g), and SF6(g) are +278.8, +79.0, 775, and... Problem 137CP: You have a l.00-mole sample of water at 30.C and you heat it until you have gaseous water at 140.C.... Problem 138CP: A 500.0-g sample of an element at 195C is dropped into an ice--water mixture; 109.5 g ice melts and... Problem 140IP: When 1.00 L of 2.00 M Na2SO4 solution at 30.0c is added to 2.00 L of 0.750 M Ba(NO3)2 solution at... Problem 141IP: The preparation of NO2(g) from N2(g) and O2(g) is an endothermic reaction: N2(g) + O2(g) NO2(g)... Problem 142IP: Nitromethane, CH3NO2, can be used as a fuel. When the liquid is burned, the (unbalanced) reaction is... Problem 143IP: A cubic piece of uranium metal (specific heat capacity = 0.117 J/C g) at 200.0C is dropped into... Problem 145MP: A gaseous hydrocarbon reacts completely with oxygen gas to form carbon dioxide and water vapour.... Problem 110AE: Given the following data: NO2(g) NO(g) + O(g)H = 233 kJ 2O3(g) 3O2(g)H = 427 kJ NO(g) + O3(g) ...
Related questions
Concept explainers
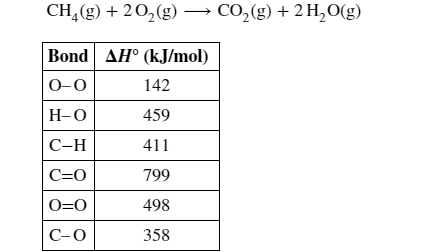
23 Calculate ΔH ° for the reaction using the given bond dissociation energies.
Transcribed Image Text: CH,(g) + 20,(g)→ CO,(g) + 2 H,0(g)
Bond AH° (kJ/mol)
0-0
142
H-0
459
С-Н
411
C=0
799
O=0
498
С-О
358
Formula Formula Bond dissociation energy (BDE) is the energy required to break a bond, making it an endothermic process. BDE is calculated for a particular bond and therefore consists of fragments such as radicals since it undergoes homolytic bond cleavage. For the homolysis of a X-Y molecule, the energy of bond dissociation is calculated as the difference in the total enthalpy of formation for the reactants and products. X-Y → X + Y BDE = Δ H f X + Δ H f Y – Δ H f X-Y where, ΔHf is the heat of formation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images