(CH 12 HW Exercise 12.66 with feedback Rev One brand of laundry bleach is an aqueous solution containing 4.00% sodium hypochlorite (NaOCI) by mass. Part A You may want to reference (Page) section 12.5 while completing this problem. What is the molarity of this solution? (Assume a density of 1.02 g/mL.) Express your answer in molarity. να ΑΣφ Request Answer Submit Provide Feedback P Pearson Permissions Contact Privacy Policy Terms of Use Copyright © 2020 Pearson Education Inc. All rights reserved. arch 112 f9 fg f6 15 f4 & L. 3 %24 MI
(CH 12 HW Exercise 12.66 with feedback Rev One brand of laundry bleach is an aqueous solution containing 4.00% sodium hypochlorite (NaOCI) by mass. Part A You may want to reference (Page) section 12.5 while completing this problem. What is the molarity of this solution? (Assume a density of 1.02 g/mL.) Express your answer in molarity. να ΑΣφ Request Answer Submit Provide Feedback P Pearson Permissions Contact Privacy Policy Terms of Use Copyright © 2020 Pearson Education Inc. All rights reserved. arch 112 f9 fg f6 15 f4 & L. 3 %24 MI
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 71GQ: An aqueous solution containing 10.0 g of starch per liter has an osmotic pressure of 3.8 mm Hg at 25...
Related questions
Question
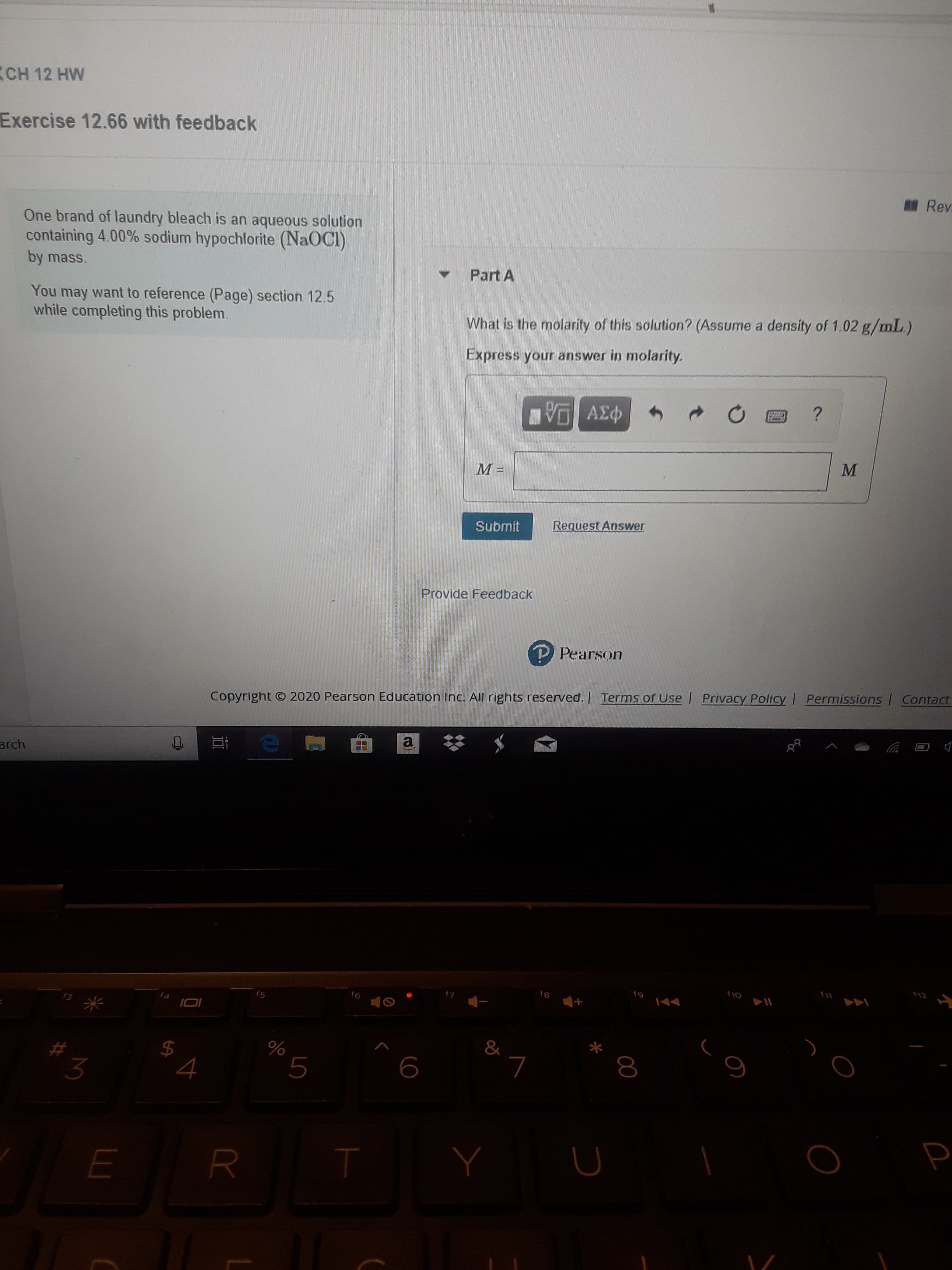
Transcribed Image Text:(CH 12 HW
Exercise 12.66 with feedback
Rev
One brand of laundry bleach is an aqueous solution
containing 4.00% sodium hypochlorite (NaOCI)
by mass.
Part A
You may want to reference (Page) section 12.5
while completing this problem.
What is the molarity of this solution? (Assume a density of 1.02 g/mL.)
Express your answer in molarity.
να ΑΣφ
Request Answer
Submit
Provide Feedback
P Pearson
Permissions Contact
Privacy Policy
Terms of Use
Copyright © 2020 Pearson Education Inc. All rights reserved.
arch
112
f9
fg
f6
15
f4
&
L.
3
%24
MI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning