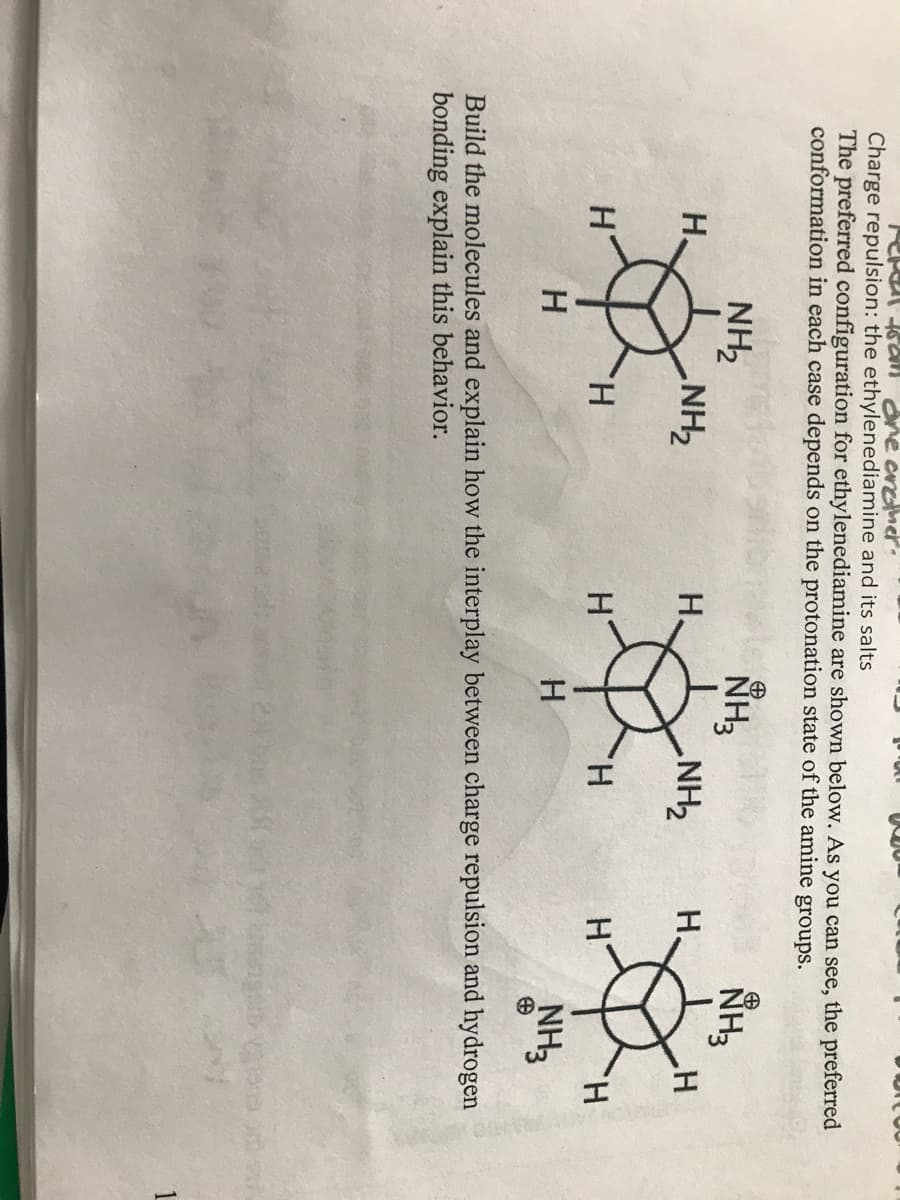
Charge repulsion: the ethylenediamine and its salts The preferred configuration for ethylenediamine are shown below. As you can see, the preferred conformation in each case depends on the protonation state of the amine groups. NH3 NH3 NH2 NH2 H. NH2 H. H. H. H' H. H H. H H NH3 Build the molecules and explain how the interplay between charge repulsion and hydrogen bonding explain this behavior.
Charge repulsion: the ethylenediamine and its salts The preferred configuration for ethylenediamine are shown below. As you can see, the preferred conformation in each case depends on the protonation state of the amine groups. NH3 NH3 NH2 NH2 H. NH2 H. H. H. H' H. H H. H H NH3 Build the molecules and explain how the interplay between charge repulsion and hydrogen bonding explain this behavior.
Chapter20: Carboxylic Acids And Nitriles
Section20.SE: Something Extra
Problem 20VC: Electrostatic potential maps of anisole and thioanisole are shown. Which do you think is the...
Related questions
Question
Transcribed Image Text:n ane arther.
Charge repulsion: the ethylenediamine and its salts
The preferred configuration for ethylenediamine are shown below. As you can see, the preferred
conformation in each case depends on the protonation state of the amine groups.
NH3
NH2
NH2
H.
NH3
H.
H.
ZHN'
H.
H.
H.
H
H
NH3
Build the molecules and explain how the interplay between charge repulsion and hydrogen
bonding explain this behavior.
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



