Chem 152 Homework #7 Aqua regia is prepared by mixing a nitric acid solution with a hydrochloric acid solution. The resulting mixture is very corrosive and is used to react with things that normally won't react with other molecules. It is used in specialized metal refining situations due to the fact that it can dissolve gold and platinum metals-something that can generally not be done otherwise. When this solution is prepared, a variety of chemical reactions occur which provide the reactive power of the solution. The most important reaction is: 2 HNO3(g) + 6 HCl(g) → 2 NO(g) + 3 Cl2(g) + 4 H2O(1) AHxn= -141.16 kJ a. If 10.0 g of HCl and 75.0 g of HNO3 are mixed and allowed to react, which reactant is the limiting reagent? 0.274 moles HEI 1.141 moles H3 b. Given your answer to part a., determine how many grams of nitric oxide (NO) should be produced? C. Given your answer to part a, how much thermal energy (heat) would be absorbed (or generated) in the reaction of 10.0 g HNO3 ? d. Is this reaction endothermic or exothermic? Which are more stable (i.e., at a HCl and 3.00 moles of lower energy) the reactants or products? I mole = 2.7436g NO 30.01 a) Helx 10y 36.46 Hel 6 I moe NO I mole 63.02 HND3 36.01 2. E 35.1g NO 759HNO3 x Imole NO 4) Hel is me imting engont ) 2.7y NO pisdueed piodoced
Chem 152 Homework #7 Aqua regia is prepared by mixing a nitric acid solution with a hydrochloric acid solution. The resulting mixture is very corrosive and is used to react with things that normally won't react with other molecules. It is used in specialized metal refining situations due to the fact that it can dissolve gold and platinum metals-something that can generally not be done otherwise. When this solution is prepared, a variety of chemical reactions occur which provide the reactive power of the solution. The most important reaction is: 2 HNO3(g) + 6 HCl(g) → 2 NO(g) + 3 Cl2(g) + 4 H2O(1) AHxn= -141.16 kJ a. If 10.0 g of HCl and 75.0 g of HNO3 are mixed and allowed to react, which reactant is the limiting reagent? 0.274 moles HEI 1.141 moles H3 b. Given your answer to part a., determine how many grams of nitric oxide (NO) should be produced? C. Given your answer to part a, how much thermal energy (heat) would be absorbed (or generated) in the reaction of 10.0 g HNO3 ? d. Is this reaction endothermic or exothermic? Which are more stable (i.e., at a HCl and 3.00 moles of lower energy) the reactants or products? I mole = 2.7436g NO 30.01 a) Helx 10y 36.46 Hel 6 I moe NO I mole 63.02 HND3 36.01 2. E 35.1g NO 759HNO3 x Imole NO 4) Hel is me imting engont ) 2.7y NO pisdueed piodoced
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter21: The Chemistry Of The Main Group Elements
Section: Chapter Questions
Problem 118IL
Related questions
Question
I need help with c)

Transcribed Image Text:Chem 152 Homework #7
Aqua regia is prepared by mixing a nitric acid solution with a hydrochloric acid solution.
The resulting mixture is very corrosive and is uscd to react with things that normally won't
react with other molecules. It is used in specialized metal refining situations due to the fact
that it can dissolve gold and platinum metals- something that can generally not be done
otherwise.
When this solution is prepared, a variety of chemical reactions occur which provide the
reactive power of the solution. The most important reaction is:
2 HNO3(g) + 6 HCl(g) → 2 NO(g) + 3 Cl2(g) + 4 H2O(1)
AHrxn = -141.16 kJ
a. If 10.0 g of HCl and 75.0 g of HNO3 are mixed and allowed to react, which
reactant is the limiting reagent?
0.274 moles HCl
1.191 moles HAD3
b. Given your answer to part a., determine how many grams of nitric oxide (NO)
should be produced?
C. Given your answer to part a, how much thermal energy (heat) would be
absorbed (or generated)
the reaction of
0.0 g of HCl and 3.00 moles of
HNO3 ?
d. Is this reaction endothermic or exothermic? Which are more stable (i.e., at a
lower energy) the reactants or products?
I mole
36.46 Hel
30.01
= 2.7436g
NO
HClx
a) 10y
I mole NO
36.01.
E 35.11g No
I mole
Imole NO
759HND3 * 63.02 Hms 2
)2.7y NO pladoeced
4) Hel is me imting engont 2.7y NO plodueed
Expert Solution
Step 1
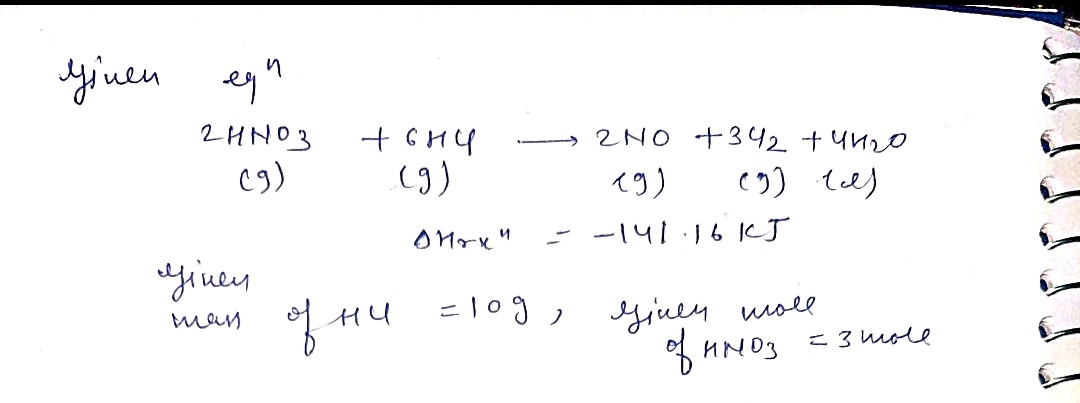
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax