Is the following reaction spontaneous when all of the solutes are in their standard states? 4Ag°(aq) + 4OH (aq) 4Ag(s) + 0,(g) + 2H,O(1) Half-Reaction E° (V) F2(8) + 2e¯ = 2F¯(aq) Cl2(g) + 2e¯ = 201"(aq) MnO2(s) + 4H*(aq) + 2e¯ = Mn²*(aq) + 2H2O(1) NO, (aq) + 4H*(aq) + 3e¯ = NO(g) + 2H¿O(1) Ag"(aq) + e = Ag(s) Fe*(aq) + e¯ = Fe²*(aq) O,(g) + 2H2O(1) + 4e¯ → 40H¯ (aq) Cu* (aq) + 2e¯ Cu(s) 2H*(aq) + 2e¯ (g) N2(g) + 5H*(aq) + 4e¯ N,H5*(aq) Fe2*(aq) + 2e¯ Fe(s) 2H,O() + 2e = H2(g) + 20H¯(aq) Na" (aq) + e¯ = Na(s) Li*(aq) + e¯ =► Li(s) +2.87 +1.36 +1.23 +0.96 +0.80 +0.77 +0.40 +0.34 0.00 -0.23 -0.44 -0.83 -2.71 -3.05 А. Yes В. No
Is the following reaction spontaneous when all of the solutes are in their standard states? 4Ag°(aq) + 4OH (aq) 4Ag(s) + 0,(g) + 2H,O(1) Half-Reaction E° (V) F2(8) + 2e¯ = 2F¯(aq) Cl2(g) + 2e¯ = 201"(aq) MnO2(s) + 4H*(aq) + 2e¯ = Mn²*(aq) + 2H2O(1) NO, (aq) + 4H*(aq) + 3e¯ = NO(g) + 2H¿O(1) Ag"(aq) + e = Ag(s) Fe*(aq) + e¯ = Fe²*(aq) O,(g) + 2H2O(1) + 4e¯ → 40H¯ (aq) Cu* (aq) + 2e¯ Cu(s) 2H*(aq) + 2e¯ (g) N2(g) + 5H*(aq) + 4e¯ N,H5*(aq) Fe2*(aq) + 2e¯ Fe(s) 2H,O() + 2e = H2(g) + 20H¯(aq) Na" (aq) + e¯ = Na(s) Li*(aq) + e¯ =► Li(s) +2.87 +1.36 +1.23 +0.96 +0.80 +0.77 +0.40 +0.34 0.00 -0.23 -0.44 -0.83 -2.71 -3.05 А. Yes В. No
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter17: Chemcial Thermodynamics
Section: Chapter Questions
Problem 17.11QE: Explain why absolute entropies can be measured.
Related questions
Question
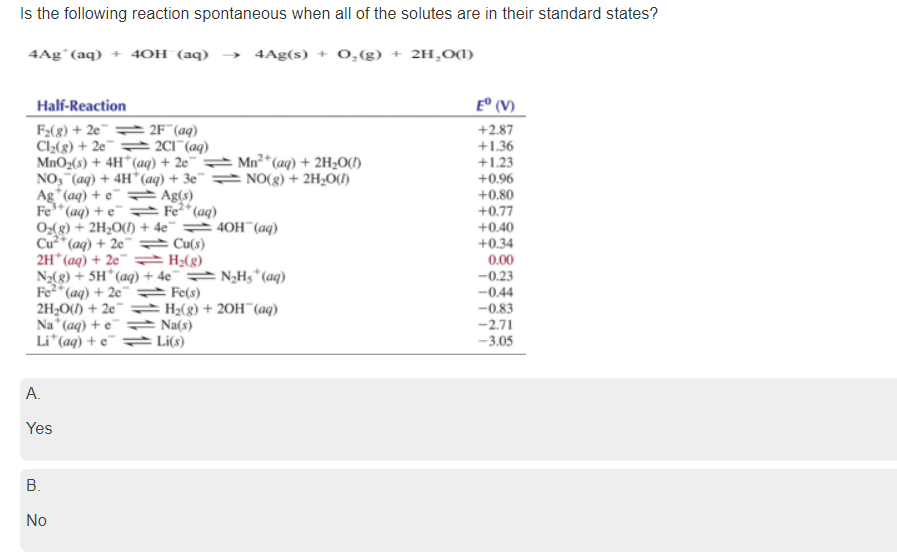
Transcribed Image Text:Is the following reaction spontaneous when all of the solutes are in their standard states?
4Ag°(aq) + 4OH (aq)
4Ag(s) + 0,(g) + 2H,O(1)
Half-Reaction
E° (V)
F2(8) + 2e¯ = 2F¯(aq)
Cl2(g) + 2e¯ = 201"(aq)
MnO2(s) + 4H*(aq) + 2e¯ = Mn²*(aq) + 2H2O(1)
NO, (aq) + 4H*(aq) + 3e¯ = NO(g) + 2H¿O(1)
Ag"(aq) + e = Ag(s)
Fe*(aq) + e¯ = Fe²*(aq)
O,(g) + 2H2O(1) + 4e¯ → 40H¯ (aq)
Cu* (aq) + 2e¯ Cu(s)
2H*(aq) + 2e¯ (g)
N2(g) + 5H*(aq) + 4e¯ N,H5*(aq)
Fe2*(aq) + 2e¯ Fe(s)
2H,O() + 2e = H2(g) + 20H¯(aq)
Na" (aq) + e¯ = Na(s)
Li*(aq) + e¯ =► Li(s)
+2.87
+1.36
+1.23
+0.96
+0.80
+0.77
+0.40
+0.34
0.00
-0.23
-0.44
-0.83
-2.71
-3.05
А.
Yes
В.
No
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning