ifa system is performing 45 Jof work to the surrounding and is gaining 30 J of heat from the surrounding, which o shows the correct signs for heat, work and change in internal energy (AU) representing the system? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. q = +, w = +, AU = + q = -,W = ,AU = – q= +,w - -, AU = + q=-,w= +, AU = + q =+,w= =,AU = – q= -, w =+,AU = –
ifa system is performing 45 Jof work to the surrounding and is gaining 30 J of heat from the surrounding, which o shows the correct signs for heat, work and change in internal energy (AU) representing the system? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. q = +, w = +, AU = + q = -,W = ,AU = – q= +,w - -, AU = + q=-,w= +, AU = + q =+,w= =,AU = – q= -, w =+,AU = –
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 10P
Related questions
Question
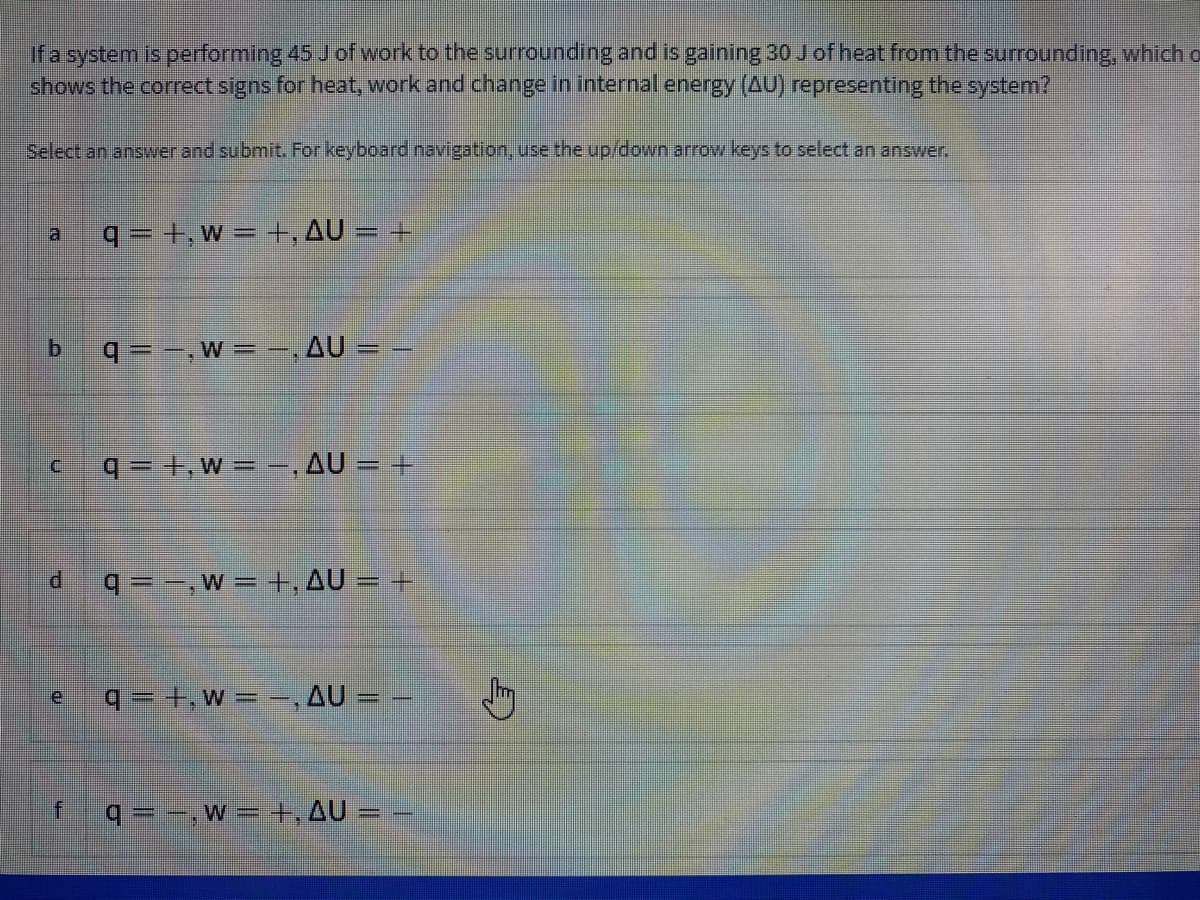
Transcribed Image Text:ifa system is performing 45 Jof work to the surrounding and is gaining 30 J of heat from the surrounding, which o
shows the correct signs for heat, work and change in internal energy (AU) representing the system?
Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer.
q = +, w = +, AU = +
q = -,W = ,AU = –
q= +,w - -, AU = +
q=-,w= +, AU = +
q =+,w= =,AU = –
q= -, w =+,AU = –
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning