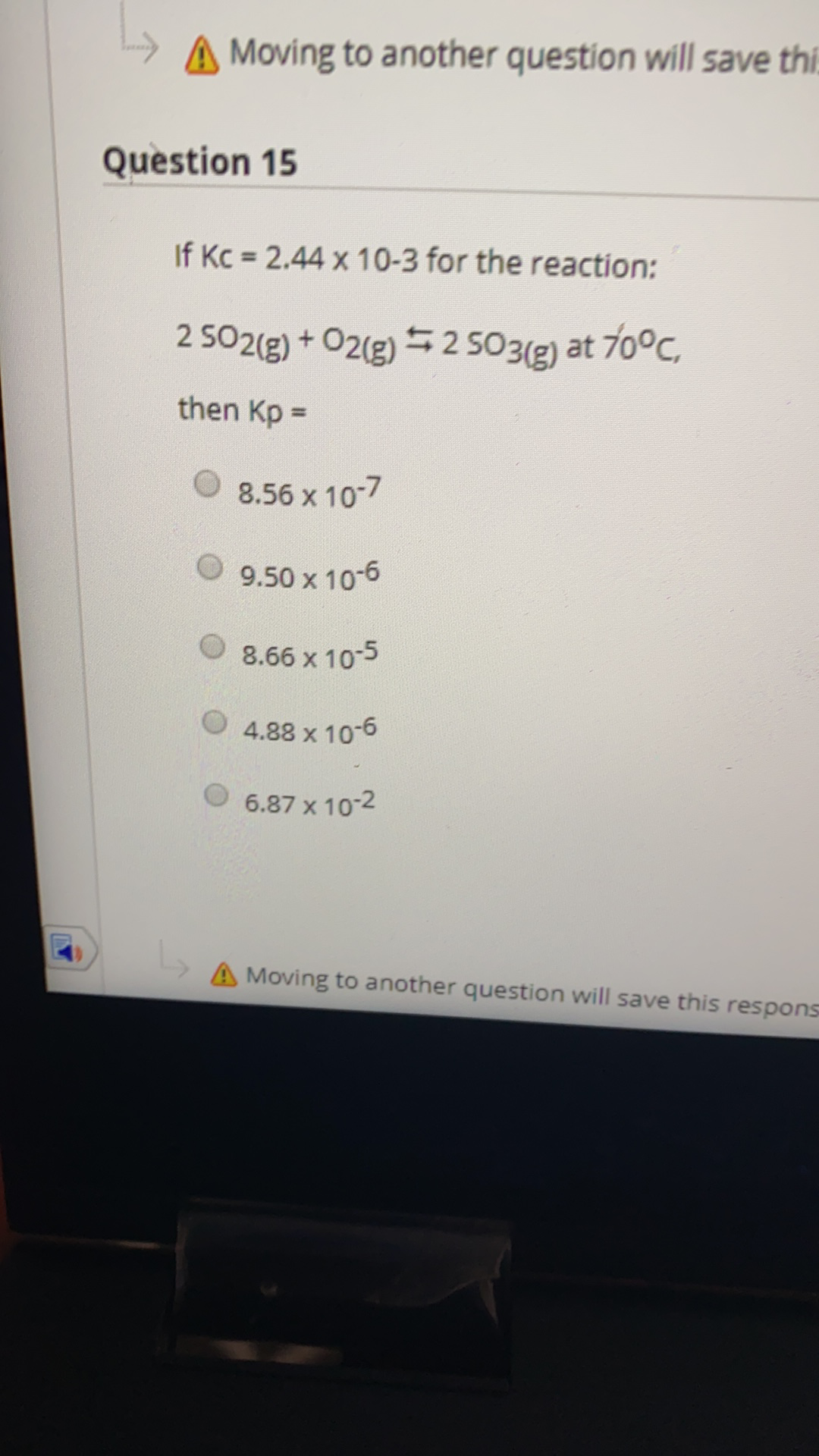
A Moving to another question will save thi. Question 15 If Kc 2.44 x 10-3 for the reaction: 2 502(g) + O2(g) = 2 503(g) at 70°C, then Kp = 8.56 x 10-7 9.50 x 10-6 O 8.66 x 10-5 4.88 x 10-6 6.87 x 10-2 A Moving to another question will save this respons
A Moving to another question will save thi. Question 15 If Kc 2.44 x 10-3 for the reaction: 2 502(g) + O2(g) = 2 503(g) at 70°C, then Kp = 8.56 x 10-7 9.50 x 10-6 O 8.66 x 10-5 4.88 x 10-6 6.87 x 10-2 A Moving to another question will save this respons
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.27PAE
Related questions
Question
Transcribed Image Text:A Moving to another question will save thi.
Question 15
If Kc 2.44 x 10-3 for the reaction:
2 502(g) + O2(g) = 2 503(g) at 70°C,
then Kp =
8.56 x 10-7
9.50 x 10-6
O 8.66 x 10-5
4.88 x 10-6
6.87 x 10-2
A Moving to another question will save this respons
Expert Solution
Step 1
Relation between Kp and Kc:
Kp = Kc (RT)∆n
Where, ∆n = Number of product molecules - Number of reactant molecules
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning