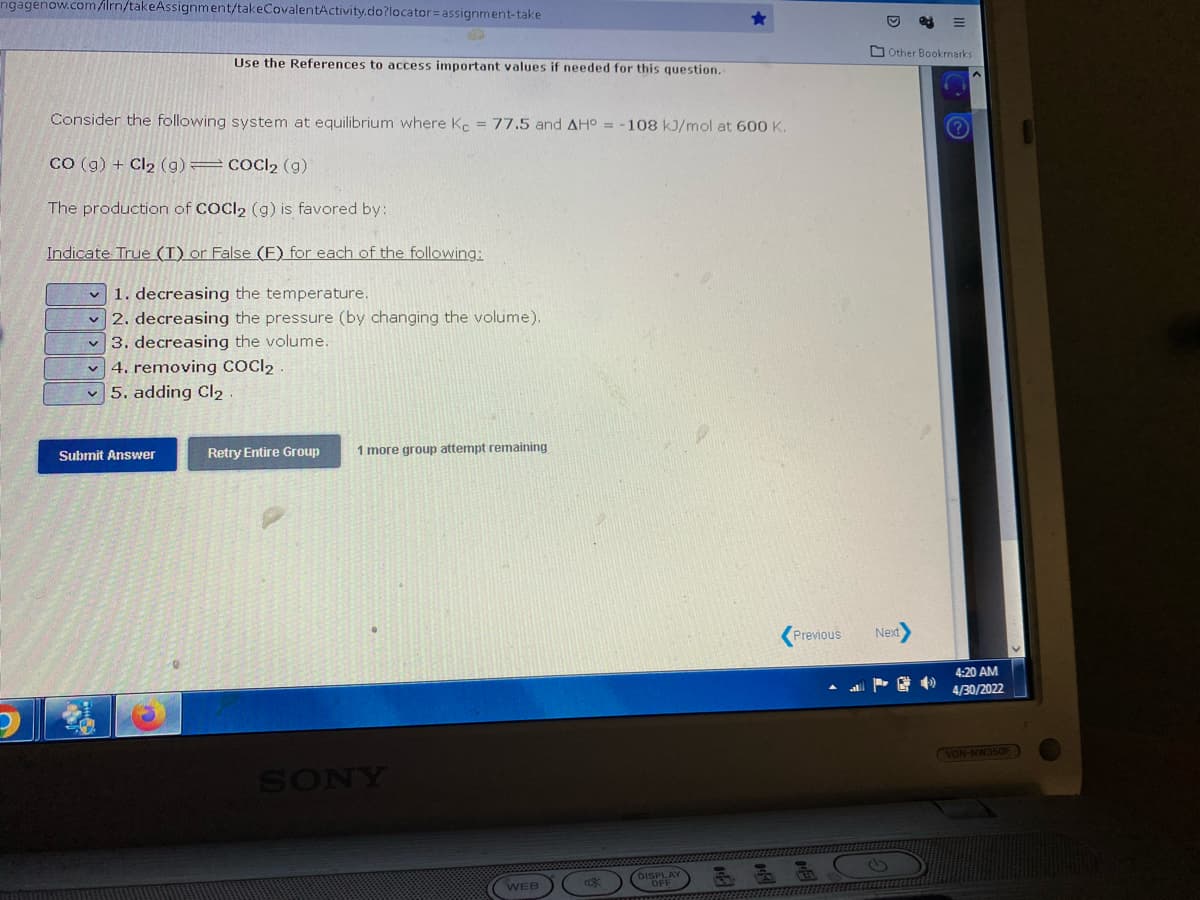
Consider the following system at equilibrium where Kc = 77.5 and AH° = -108 kJ/mol at 600 K. co (g) + Cl₂ (g) = COCl₂ (g) The production of COCI₂ (g) is favored by: Indicate True (I) or False (F) for each of the following: ✓1. decreasing the temperature. 2. decreasing the pressure (by changing the volume). 3. decreasing the volume. 4. removing COCI₂. 5. adding Cl₂.
Consider the following system at equilibrium where Kc = 77.5 and AH° = -108 kJ/mol at 600 K. co (g) + Cl₂ (g) = COCl₂ (g) The production of COCI₂ (g) is favored by: Indicate True (I) or False (F) for each of the following: ✓1. decreasing the temperature. 2. decreasing the pressure (by changing the volume). 3. decreasing the volume. 4. removing COCI₂. 5. adding Cl₂.
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 7RQ: If you calculate a value for G for a reaction using the values of Gf in Appendix 4 and get a...
Related questions
Question
Transcribed Image Text:ngagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator= assignment-take
Use the References to access important values if needed for this question..
Consider the following system at equilibrium where Kc = 77.5 and AH° = -108 kJ/mol at 600 K.
CO(g) + Cl₂ (g) COCI2 (g)
The production of COCl₂ (g) is favored by:
Indicate True (I) or False (F) for each of the following:
✓1. decreasing the temperature.
✓2. decreasing the pressure (by changing the volume).
3. decreasing the volume.
4. removing COCI2
5. adding Cl₂
Retry Entire Group
1 more group attempt remaining
Previous
Submit Answer
SONY
WEB
OFF
Ⓒ
E
Other Bookmarks
Next
4:20 AM
4/30/2022
VGN-NW350F
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning