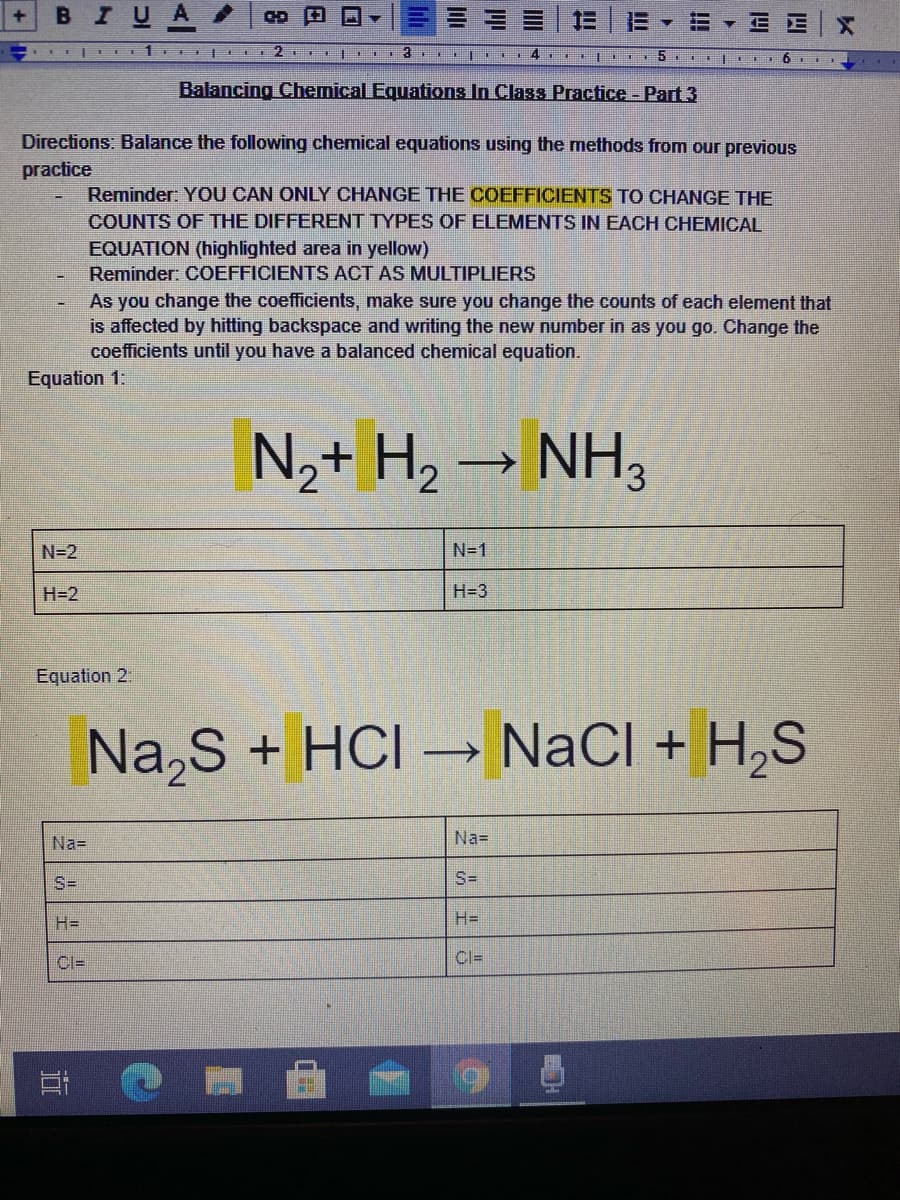
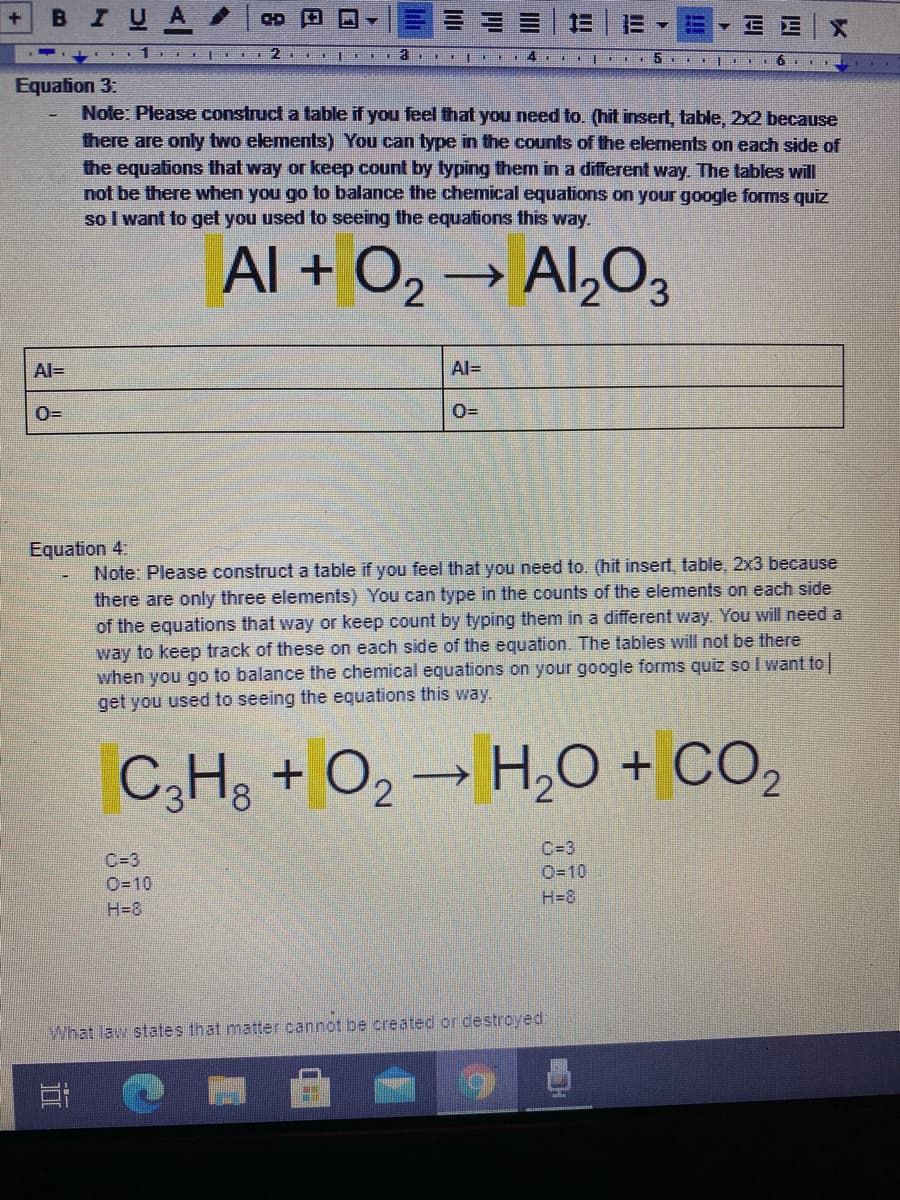
в IUA 三,=▼EE|X 5 Balancing Chemical Equations In Class Practice - Part 3 Directions: Balance the following chemical equations using the methods from our previous practice Reminder: YOU CAN ONLY CHANGE THE COEFFICIENTS TO CHANGE THE COUNTS OF THE DIFFERENT TYPES OF ELEMENTS IN EACH CHEMICAL EQUATION (highlighted area in yellow) Reminder: COEFFICIENTS ACT AS MULTIPLIERS As you change the coefficients, make sure you change the counts of each element that is affected by hitting backspace and writing the new number in as you go. Change the coefficients until you have a balanced chemical equation. Equation 1: N,+ H, → NH, N=2 N=1 H=2 H=3 Equation 2. Na,S + HCI → NaCl + H,S Na= Na= S= S= H3D H= CI= Cl= BIUA CD 5 Equation 3: Note: Please construct a table if you feel that you need to. (hit insert, table, 2x2 because there are only two elemets) You can type in the counts of the elements on each side of the equations that way or keep count by typing them in a different way. The tables will not be there when you go to balance the chemical equations on your google forms quiz so I want to get you used to seeing the equations this way. Al + O2→ Al,O3 Al= Al= Equation 4. Note: Please construct a table if you feel that you need to. (hit insert. table, 2x3 because there are only three elements) You can type in the counts of the elements on each side of the equations that way or keep count by typing them in a different way. You will need a way to keep track of these on each side of the equation. The tables will not be there when you go to balance the chemical equations on your google forms quiz so I want to get you used to seeing the equations this way. C,H, + O2 → H,0 + CO, C=3 C=3 0-10 O=10 H=8 H=8 What law states that matter cannot be created or destroyed.
States of Matter
The substance that constitutes everything in the universe is known as matter. Matter comprises atoms which in turn are composed of electrons, protons, and neutrons. Different atoms combine together to give rise to molecules that act as a foundation for all kinds of substances. There are five states of matter based on their energies of attraction, namely solid, liquid, gases, plasma, and BEC (Bose-Einstein condensates).
Chemical Reactions and Equations
When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. These reactions are best explained using a chemical equation.
Step by step
Solved in 2 steps with 1 images









